Cholinergic drugs (Cholinomimetic, Para-sympathomimetic)
· The drugs which produced action similar to that of the Acetyl choline (Ach), either by interacting with cholinergic receptor (Cholinergic antagonist) or by increasing availability of Ach at these site (Anti-cholinesterase)
Cholinergic transmission
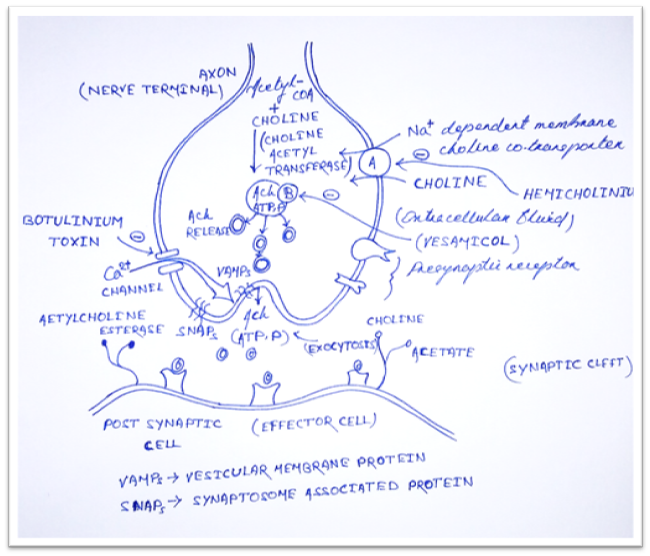
1) Synthesis
· Ach is synthesized in the cytoplasm from Acetyl-CoA and choline by the catalytic action of choline acetyltransferase (ChAT).
· Acetyl-CoA is synthesized in mitochondria, which are present in large number in the nerve ending.
· Choline is transported from the extracellular fluid into the neuron terminal by Na+ dependent membrane choline cotransporter (Carrier A).
· Drugs that block this action is Hemicholinium.
2) Release
· Synthesized Ach is transported from cytoplasm into by anti-port (molecules moves in opposite direction) that removes proton (Carrier B).
· This action is blocked by Vesamicol.
· Release is dependent on extracellular Ca2+ and occur when action potential (AP) reaches the terminal and trigger sufficient influx of Ca2+ ions.
· The increase Ca2+ concentration destabilizes the storage vesicles by interacting the special protein VAMPs (vesicular membrane protein) and SNAPs (Synaptosome associated protein).
· Fusion of vesicular membrane with the terminal membrane results in exocytosis expulsion of Ach into the synaptic cleft.
· This action is blocked by Botulinum toxin.
3) Destruction
· After release: Ach molecules may bind to and activate Ach receptor (Cholino-receptor).
· All of the Ach released will diffuse with range of acetylcholinesterase (AchE).
· AchE very efficiently split Ach into choline and acetate.
· Most cholinergic synapses are richly supplied with AchE.
· Half-life of Ach in the synapse is very short.
· AchE also found in other tissue e.g. RBC
· Another cholinesterase with a lower specificity for Ach, butyryl-cholinesterase (Pseudo-cholinesterase) is found in Blood plasma, liver, glial and many other tissue.
Cholinergic receptor
· There are two types of cholinergic receptor;
1. Muscarinic receptor
2. Nicotinic receptor
Muscarinic receptor
· It is primarily located in autonomic effector cells in heart, eye, smooth muscles and gland of GIT and CNS.
Types of Muscarinic receptor

Location of muscarinic receptor
M1: Autonomic ganglion cells, gastric glands and central neurons (cortex, hippocampus, curpusstriatum)
Physiological role: mediation of gastric acid secretion and relaxation of LES (Vagal)
Learning, memory and motor function.
M2: cardiac muscarinic receptors.
Physiological role: mediated vagal bradycardia
Auto receptor in cholinergic nerve endings
CNS-Tremor, analgesia
M3: Visceral smooth muscles, glands and vascular endothelium. Also Iris and ciliary muscles.
Nicotinic (N) receptors
· Nicotinic action of Ach are those that can be reproduced by the injection of nicotine (Nicotiana tabacum)
· Can be blocked by tubocurarine and hexamethonium
· Activation results in a rapid increase in cellular permeability to Na+ and Ca2+ resulting depolarization and initiation of action potential.
· Further nicotinic receptor divided into NM and NN receptor.
NM (Muscular type) | NN (Ganglion type) |
· Location: Skeletal muscle end plates · Function: Stimulate skeletal muscle (Contraction). · MOA: Post synaptic and excitatory (opening of Na+ K+) · Agonist: Ach, Carbachol suxamethonium. Selectively by phenyl trimethyl ammonium (PTMA) · Antagonist: Tubocurarine, Atracurinium, Vecurinium and Pancurinium. | · Location: In autonomic ganglia of all type (ganglion type) sympathetic, parasympathetic and also adrenal medulla. · Function: depolarization and post ganglionic impulse: stimulate all the autonomic ganglia. · Adrenal medulla—catecholamine release. · MOA: opening of Na+ K+ and Cl– channel. · Agonists: Ach, cch etc. · Selectively stimulated by dimethyl phenyl piperazinium (DMPP) · Antagonists: Trimethophan, mecamylamine and hexamethonium. |
Classification
Choline ester | Alkaloids |
Natural: Acetylcholine (Ach) Synthetic: Methacholine, Carbachol and bethanicol
| Pilocarpine Muscarine Arecholine Synthetic: Oxotremorine |
Acetylcholine
Pharmacological action
- Heart (M2)
- SA node hyperpolarization: Bradycardia (decrease impulse, decrease rate of diastolic depolarization, K+ out flux, Cl– influx).
- A-V node and His-purkinje fibres refractory period (PF-RP) is increased and conduction is slow.
- PR interval increased and partially or complete heart block.
- Arterial fibres: decrease of contraction and RP in atrial fibre is abbreviated.
- Arterial fibrillation and flutter: predisposing
- Decrease in ventricular contractility (less prominent).
- Blood vessel (M3)
- M3 is present in all types of blood vessel.
- Fall in BP and flushing (A redness of skin).
- Vasodilatation by Nitric oxide (NO) release (PLC-IP3/DAG).
- Penile erection.
- Smooth muscle (M3-contracted)
- Abdominal cramps, diarrhoea due to increased peristalsis and relaxed sphincters.
- Bronchial smooth muscle contraction: dyspnoea (difficulty in breathing).
- Voiding of bladder (incomplete relaxation of bladder muscle and urethra).
- Gland (M3)
- Increase secretion [sweating, salivation, lacrimation, tracheobronchial tree (damage the airway and bronchi) and gastric gland].
- Eye (M3)
- Contraction of circular fibres of iris (Miosis).
- Contraction of ciliary muscles.
Nicotinic
- Autonomic ganglia
- Both sympathetic and parasympathetic ganglia are stimulated.
- After atropine injection Ach causes tachycardia and increase in BP.
- Skeletal muscle
- V. injection – no effect
- Contraction of skeletal muscle
- CNS
- Does not penetrate blood brain barrier (BBB).
- Local injection in CNS – Complex action.
Interaction
- Anticholinesterase potentiates markedly, methanicol to less extend and have only additive action with Carbachol or bethanicol, depending upon the role of ChE in the termination of action of the particular choline ester.
- Atropine and its congeners competitively antagonize muscarinic actions.
- Acetylcholine is not used therapeutically-nonspecific.
Bethanicol
- Used in postoperative or postpartum non-obstructive urinary retention, neurogenic bladder to promote urination, congenital megacolon and GERD.
- Side effect: belching, colic, involuntary urination/defecation, flushing, sweating, fall in BP, bronchospasm.
- Dose: 10-40mg oral, 2.5-5mg S.C. (Urotonin, Bethacol, 25mg tab.)
Pilocarpine
- Alkaloid from leaves of jaborandi (Pilocarpus microphyllus)
- Prominent muscarinic actions and also stimulate ganglia (ganglionic muscarinic receptor).
- Caused marked sweating, salivation, lacrimation and other secretion.
- Dilate blood vessels (Hypotension).
- High dose: increase BP and tachycardia (ganglionic action)
- On eye: produce miosis and spasm of accommodation.
- Lower intraocular pressure (IOP) in glaucoma.
- CNS toxicity.
- Diaphoretic (excessive sweating)
- Xerostomia (Dryness of mouth)
- Sjogren’s syndrome (dry mouth, dry eyes)
- To reverse mydriasis effect of atropine.
- To break adhesion between iris and cornea/lens alternated with mydriatic.
- Atropine is used as antidote in acute pilocarpine poising (1-2 mg I.V. 8 hourly).
- Dose: Pilocarpine eye drop 0.5-4 %.
Pilocarpine in glaucoma
- Construction of circular muscle of Iris.
- Construction of ciliary muscle.
- Spam of accommodation-fixed at near vision.
Muscarine
- Alkaloid from mushroom Amanita muscaria.
- It has only muscarinic action.
- No clinical use.
- Mushroom poisoning due to ingestion of poisonous mushroom.
- Early onset mushroom poisoning (Muscarine type)
- Late onset mushroom poisoning.
- Hallucinogenic type.
Anti-cholinesterase
- Anti-cholinesterase (Anti-ChEs) are agent which inhibit the cholinesterase that is responsible for hydrolysis of Ach. Thus Ach is not metabolized and get accumulated at muscarinic and nicotinic sites.
- Some ChEs have additional direct effect in cholinergic receptor.
- Cholinesterase: it is an enzyme that catalyzes the hydrolysis of Ach into choline and acetic acid.
Reversible
Carbamate | Acridine |
· Physostigmine · Neostigmine · Pyridostigmine · Galantamine · Edrophomium · Rivastigmine · Donepezil
| · Tracaine |
Irreversible
Organophosphate | Carbamate |
· Dyflos · Echothiophate · Parathion, Malathion, Diazinone · Tabun, sarin, soman | · Propoxur · Carbaryl |
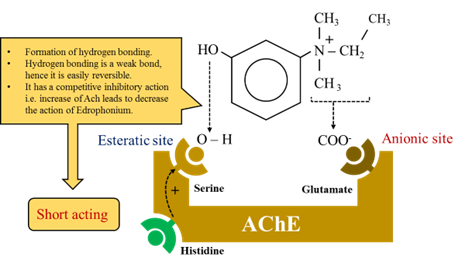
Mechanism of action
- The active site of acetylcholine is made up of two sub sites.

Reversible
· Acetylcholinesterase inhibitor (anticholinesterase agents or cholinesterase inhibitors) indirectly provide a cholinergic action by preventing the degradation of Ach.
· This results accumulation of Ach in the synaptic cleft and provoke to bind with cholinoreceptor.
· It is classified as short-acting or intermediate acting agents.
Irreversible
· A number of synthetic organophosphorus compounds have capacity to bind covalently to AchE.
· This result is a long lasting increase in Ach at site where it is released.
N.B: Carbamate – Carbamylate is the active site of the enzyme.
· Carbamate fuse covalently with serine.
· Carbamylated (Reversible inhibitor) reacts with water slowly and the Esteratic site is freed and ready for action-30min (less than synthesis of fresh enzyme).
Phosphate – Phosphorylate the enzyme.
· Phosphorylated (irreversible) reacts extremely slowly or not at all (take more time then synthesis of fresh enzyme).
· Sometimes phosphorylated enzyme loses one alkyl group and become resistant to hydrolysis – aging.
· Endophonium and tacrine react only at anionic site. Short acting organophosphates react only at Esteratic site.
Pharmacological action
· Ganglia: stimulate ganglia – muscarinic action – less prominent action.
· CVS: Muscarinic – Bradycardia and hypotension
Ganglionic action – stimulation – increase HR and BP, medullary center – stimulation followed by depression – overall unpredictable.
· Skeletal muscle: increase force of contraction in muscle.
Reversible
Physostigmine
· Psysiostigmine is a nitrogenous cabamic acid ester found naturally in plants and tertiary amine.
· It is stable carbamoylated intermediate with the enzyme and reversibly inactivated.

Action
· Stimulate muscarinic, nicotinic, nicotinic receptor of the NMJ.
· Duration of action 2-4 hours (intermediate acting.
Therapeutic uses
· Increases intestinal and bladder motility.
· Miosis or spasm of accommodation (ciliary muscle of the eye remain in a constant state).
· Reduce IOP (less effective than pilocarpine).
· Used as overdose of atropine, phenothiazine, and tricycle antidepressant.
Averse effect
· Convulsion (high dose)
· Bradycardia
· Accumulation of Ach at skeletal NMJ, results in paralysis.
Neostigmine
· Similar action that Psysiostigmine.
Action
· Neostigmine has quaternary nitrogen, therefore it is more polar, so is absorbed poorly from GI tract and does not enter the CNS.
· Greater effect on skeletal muscle.
· Duration of action 30 min to 2 hours (intermediate)
Therapeutic uses
· Salivation
· Flushing decrease BP
· Nausea
· Abdominal pain
· Diarrhea
· Bronchospasm
· Does not cause CNS side effect.
· Intestine and urinary bladder obstruction, neostigmine gets contraindicated.
· Should not used in case of peritonitis (inflammation of peritoneum) or inflammation of bowel disease.
Edrophonium
· Short acting inhibitor
· Bind reversibly to AChE, prevent the hydrolysis of Ach.
· Duration of action 10-20 min.
· Used in the diagnostic of Myasthenia gravis.
Pyridostigmine
· Used in chronic management of Myasthenia gravis.
· Duration of action 3-8 hours.
· Adverse effect similar to neostigmine.
Tacrine, Rivastigmine, Donepezil, Galantamine
· Alzheimer disease: deficiency of cholinergic neurons in the CNS.
· Development of AChEs as possible remedies for the loss of cognitive function.
· Tacrine: produce hepatotoxicity.
· Donepezil, Rivastigmine, Galantamine to delay the progression of Alzheimer disease.
· GI distress is primary adverse effect.
Irreversible
Echothiophate
MOA
· It is an organophosphorus that covalently binds via its phosphate group to serine-OH group at the active site of AChE and the enzyme permanently inactive and restoration of AChE activity requires the synthesis of new enzyme molecules.
· The phosphorylated enzyme slowly release one of its ethyl groups. The loss of an alkyl groups which is called aging.

Action
· Paralysis of motor function (breathing difficulties)
· Convulsion
· Miosis
· Decrease IOP (High dose of Atropine reverse)
Therapeutic uses
· Open-angle glaucoma
Long duration one week.

Hi…!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
About The Author
Hi...!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
