A cardiac arrhythmia is define as abnormal electrical
rhythm of the heart or abnormal impulse generation or conduction of electrical
impulses responsible for membrane depolarization.
Pathogenesis
·
The cardiac cycle
is normally initiated by an electrical discharge from SA node.
·
The atria and
ventricles then activated sequentially as electrical depolarization passes
through specialized conducting tissue.
· The sinus node acts as a pacemaker and its intrinsic rate is regulated by the autonomic nervous system.
· Vagal activity decrease the heart rate and sympathetic activity increase heart rate through cardiac sympathetic nerves and circulating catecholamine (it is produce by adrenal glands, location top of the kidneys).
Mechanism behind cardiac arrhythmias
1. Enhance/ectopic pacemaker activity
· Phase-4 depolarization may be increase.
· Increase of current injury ectopic impulse may results.
· Myocardial cells damaged by ischemia become partially depolarized.
2. After depolarization
a) Early after depolarization (EAD): EADs are frequently associated with long Q-T interval due to slow depolarization and markedly prolonged action potentials Aps.
b) Delay after depolarization (DAD): due to Ca2+ overload, a second deflection occur which may reach the threshold potential and premature action potential.

3. Reentry
· The tachycardia is initiated by an ectopic beat and sustain by a re-entry circuit. Most tachyarrhythmia are caused by re-entry.
i. Circus movement reentry
· A premature impulse, temporarily blocked in one direction by refractory tissue, makes one way transit around an obstacle (natural orifices in the heart, A-V nodal region).
· This types of reentry is often responsible for PSVT, atrial flutter and atrioventricular reciprocal rhythm in Wolff-Parkinson-white syndrome (WPW).
ii. Functional reentry
· In this types there is no fixed obstacle or pathway.
· Responsible for ventricular extra-systole, polymorphic ventricular tachycardia, atrial /ventricular fibrillation.
Mechanism of re-entry
· When two alternative pathway with different properties.
· In sinus rhythm, each impulse passes down both pathway before entering a common distal pathway.
· A premature impulse may find pathway ‘A’ open and B closed.
· ‘B’ pathway recover while the premature impulse travelling selectively down pathway ‘A’. The impulse travel retrogradely up pathway B, setting up a close loop or re-entry circuit.
· This may initiate a tachycardia that continues until the circuit is interrupted by a change in conduction rates or electrical depolarization.
iii. Fractionation of impulse
· An impulse generated early in diastole gets conducted irregularly over the atrium (i.e. move rapidly through fibres with short ERP).
· Longer ERP (partially recovered).

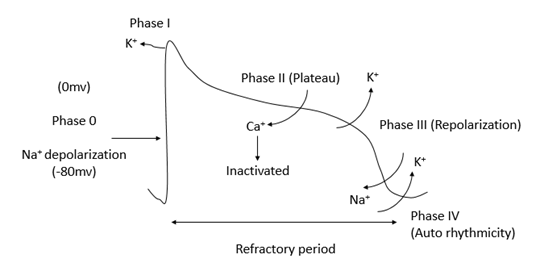
Phages of action potential of cardiac cells
1) Phase ‘0’: rapid depolarization of Na+
2) Phase ‘I’: potential depolarization phase ‘0’ (influx of Na+ current deactivated, out flow of K+).
3) Phase ‘II’: plateau (slow influx calcium current).
4) Phase ‘III’: repolarization (calcium current inactivates, K+ channel out flux).
5) Phase ‘IV’: pacemaker potential (slow Na+ influx, slowing of K+ out flux) “auto-rhythmicity”
Importance of Arrhythmia
Sinus arrhythmia
· Sinus arrhythmia does not required treatment, it occurs due to cyclical alteration of heart rate during respiration.
Sinus bradycardia
· This may commonly finding in athletes and people at rest.
· Some pathological causes are
§ Hypothermia
§ Hypothyroidism
§ Sinus node disease
§ Myocardial infraction etc.
Sinus tachycardia
· It occurs due to sympathetic activity with exercise, emotion, stress, and pregnancy, some pathological causes are:
§ Anxiety
§ Fever
§ Anemia
§ Heart failure
§ β-agonist drugs
§ thyrotoxicosis (excess thyroid hormone synthesis)

Extra-systoles
· Premature heart beats due to abnormal automaticity.
· The QRS complex in VES (Ventricular extra-systoles) is abnormal and broader in shape.
Symptoms
· Abnormal heart beating
· Anxiety
· Panic
· Sweating
· Stomach discomfort
· Headache and nausea
Causes
· Consumption of alcohol
· Physical and mental strain
· Stress
· Fatigue
· Heavy consumption of Nicotine, Noscapine
Paroxysmal supraventricular tachycardia (PSVT)
· Sudden onset episodes of atrial tachycardia (150-200/min) Wolff-Parkinson-white syndrome (WPW).
Atrial flutter (AFI)
· Atria beat at a rate of 200-350/min
AFI management
· For controlling the ventricular rate digoxin, β-blockers, or verapamil is may be used.
· β-blockers or Amiodarone can be used to prevent recurrent episodes of atrial flutter.
· Catheter ablation (it is a procedure used to terminate false electrical pathway) is highly effective against AFI.
· Anti-coagulant management may be consider.

(AFI waves are visible in II and III lead)
Ventricular tachycardia
· VT is a run of 4 or more consecutive ventricular extra systoles, it may be sustained or non-sustained arrhythmia.

(Independent atrial and ventricular activity)
· Abnormal QRS complex.
· Synchronized DC cardioversion is the treatment of choice if systolic BP is less than 90mmHg.
· If arrhythmia is well tolerated, IV amiodarone may be given as a bolus, followed by a continuous infusion.
· IV lidocaine can be used but, may depress the left ventricular function, causing hypotension or heart failure.
· Hypokalemia, hypomagnesaemia, acidosis and hypoxia should be correlated.
· β-blockers are effective.
· Class Ic should not be used, it depress the myocardial function.
· Catheter ablation is useful.
Atrial fibrillation (AF)
· Atrial fibres are activated asynchronously at a rate of about 350-550/min (due to electrophysiological inhomogenicity of atrial fibres).
· Fast ventricular rate 120-160/min.

A) Irregular QRS (fast ventricular rate)
B) Chronic critical fibrillation.
Clinical feature
· Palpitation
· Breathlessness and fatigue
· Headache
· Chest pain.
Management
· When AF complicates an acute illness such as a chest infection or pulmonary embolism occurs.
· Paroxysmal atrial fibrillation: β-blockers are used as first line therapy. Class Ic drugs, such as propafenone or flecainide are also effective at preventing episodes, but should not give to CAD or left ventricular dysfunction.
· Class III drug also useful, in case it is fail, Dronedarone is an effective alternative, but sometimes it produce contraindicate.
· Also consider catheter ablation, if drugs are not respond.
· Rhythm control: Electrical DC cardioversion (sending the electrical shock through electrode to restore normal rhythm) may be used, if the drugs were not responded.
Torsades de points
· Life threatening from polymorphic ventricular tachycardia with rapid asynchronous complex and conducting base line on ECG.
· Prolong ventricular repolarization, prolong QT interval (>0.44 sec in men, >0.46 in women).

(Long QT interval)
· IV magnesium (8mmol over 5min, than 72 mmol over 24hrs)) should be given.
Ventricular fibrillation
· Grossly irregular, rapid and fractionated activation of ventricles resulting in in-coordinated contraction of its fibres with loss of pumping function. It is fatal unless reverted within 2-5min is most common cause of sudden cardiac death.
Atrioventricular (A-V) block
· It is due depression of impulse conducting through the A-V node and bundle of His, mostly due to vagal influence or ischemia.
· First degree (A-V) bock: PR interval is prolonged (> 0.20sec)

First degree (prolonged PR interval)
· Second degree (A-V) block: Atria fails to conduct to the ventricle. Two sub types are as follow:
§ Mobitz subtype I

(PR interval progressively increased until P wave is not conducted)
§ Mobitz subtype II

(PR interval of conducted beat in normal but, some P wave are not conducted)
§ Mobitz subtype III

(Complete dissociation of atrial and ventricular complexes. The atrial and ventricular rate about 80/min and 38/min)
Classification
Class | Action | Drugs |
1 | Membrane stabilizing agent (Na+ channel blockers) |
|
A) Moderately decrease dv/dt of ‘0’ phase | Quinidine, procainamide, disopyramide, moricizine. | |
B) Little decrease dv/dt of ‘0’ phase | Lidocaine, Mexiletine | |
C) Marked decrease dv/dt of ‘0’ phase | Propafenone, Flecainide | |
2 | Anti-adrenergic agent (β-blockers) | Propranolol, Esmolol, Sotalol (also class III) |
3 | Agents widening AP (prolong repolarization and ERP | Amiodarone, Dronedarone Dofetilide, Ibutilide |
4 | Calcium channel blockers | Verapamil, diltiazem |
5 | Miscellaneous | PSVT – adenosine, digoxin AV block – atropine |
Class 1A
Quinidine
· It is obtain from cinchona bank,
· Quinidine decrease the phase “0” depolarization, prolong the action potential and increase the effective refractory period (ERP).
· Broaden the QRS complex and increases P-R and Q-T intervals.

Mechanism of action
· Quinidine blocks Na+ Channel, decrease automaticity, conduction velocity and prolong repolarization.
· Prolongation of action potential duration (APD) due to calcium channel block.
· Quinidine inactivate sodium channel and delay reactivation, other action of quinidine are decrease BP, α adrenergic blockade, and skeletal muscle contractility.
Pharmacokinetics
· Well absorbed orally
· 80% bound to plasma protein (albumin)
· Extensive hepatic oxidative metabolism
Adverse effect
· Uterine contractions,
· vomiting,
· diarrhea,
· Neurological effects like ringing in ears, vertigo, deafness, visual disturbances and mental changes (Cinchonism).
Interaction
· Metabolized by CYP450
· Increased digoxin levels
· Cardiac depression with β-blockers
· Inhibit CYP2D6
· Risk of torsades de pointes is increased by hypokalemia caused by diuretics.
Uses
· Atrial and ventricular arrhythmias
· Falciparum malaria.
Procainamide
· Slowing of 0 phase depolarization and impulse conduction, prolongation of APD, ERP, QRS complex and Q-T interval.
· It is less effective in suppressing ectopic automaticity.
· It causes less marked depression of contractility and A-V conduction.
· Antivagal action is absent.
Pharmacokinetics
· Oral bioavailability is about 75%.
· Peak plasm concentration is 1 hour.
· It is metabolized in liver, primarily by acetylation to N-acetyl procainamide (NAPA) which has no Na+ channel blocking property but blocks K+ channels and prolongs repolarization.
· Decrease APD.
· Plasm ½ life is relatively short (3-4 hours).
Dose
· For abolition of arrhythmia—0.5–1 g oral or i.m. followed by 0.25–0.5 g every 2 hours; or 500 mg i.v. loading dose (25 mg/min injection) followed by 2 mg/kg/hour.
· Maintenance dose—0.5 g every 4–6 hours.
Adverse effect
· Rashes
· Fever
· Angioedema
· Flushing and hypotension are seen on rapid i.v. injection.
· Nausea and vomiting
· Cardiac toxicity
· May cause torsades de pointes
Uses
· Procainamide is used to terminate ventricular tachycardia and some supraventricular tachycardia.
Disopyramide
· Same mechanism as quinidine but no α adrenergic blocking property.
· Prolongation of P-R interval and QRS broadening are less marked.
Pharmacokinetics
· Oral bioavailability is about 80%.
· It is partly metabolized in liver by dealkylation.
· Nearly half is excreted unchanged in urine
· Plasma t½ is 6–8 hrs.
· The t½ is increased in patients of MI and in renal insufficiency.
Dose
· 100–150 mg 6–8 hourly oral.
Adverse effect
· Dry mouth
· Constipation
· Urinary retention
· Blurred vision
· Less G.I. effects
· Increases peripheral resistance hence, chances of heart damage.
· Hypotension
Class 1B
· In this class of drugs rapid association and dissociation of sodium channel thus, the actions of class IB agents are manifested when the cardiac cell is depolarized or firing rapidly.
· Also block or inactivation of Na+ channel.

(Shorten phase III repolarization and decrease the duration of action potential)
Mechanism of action
· Lidocaine and Mexiletine shorten phase 3 repolarization and decrease the duration of the action potential.
Pharmacokinetics
· Lidocaine has extensively fast pass transformation by liver so, it is given intravenously (last only 10-20min).
· Inactive orally.
· It is hydrolysed, deethylated and conjugated; metabolites are excreted in urine.
· The t½ of early distribution phase is 8 min while that of later elimination phase is nearly 2 hours.
· Its t½ is prolonged in CHF, because of decrease in volume of distribution and hepatic blood flow.
Dose and preparation
· Lidocaine is given only by i.v. route: 50–100 mg bolus followed by 20–40 mg every 10–20 min or 1–3 mg/min infusion.
· Mexiletine 100–250 mg i.v. over 10 min., 1 mg/min infusion. Oral: 150–200 mg TDS with meals.
Therapeutic uses
· Lidocaine it is used to suppress VT and prevent VF,
· Lidocaine is safe drugs when it is given sloe i.v.
· In the emergency setting, e.g. arrhythmias following acute MI or during cardiac surgery.
· Parenteral Mexiletine may be used in post-infarction sinister ventricular arrhythmias as alternative to lidocaine.
· Oral use to chronically suppress VES/VT.
Adverse effect
· Lidocaine has wide therapeutic index and shows little impairment of left ventricular function and has no negative inotropic effect.
· Central nervous system (CNS) effects include nystagmus (early indicator of toxicity), drowsiness, slurred speech, paresthesia, agitation, confusion, and convulsions.
· Mexiletine has a narrow therapeutic index.
· Nausea, vomiting, and dyspepsia are the most common adverse effects.
Class 1C, Flecainide, Propafenone
· These are the most potent Na+ channel blockers with more prominent action on open state and the longest recovery times (> 10S).
· They markedly delay conduction, prolong P-R interval, broaden QRS complex, but have variable effect on APD.
· Drugs of this subclass have high proarrhythmic potential when administered chronically; sudden deaths have occurred.

(Markedly slow phase “0” depolarization)
Mechanism of action
· Flecainide suppresses phase 0 upstroke in Purkinje and myocardial fibers.
· This causes marked slowing of conduction in all cardiac tissue, with a minor effect on the duration of the action potential and refractoriness.
· Automaticity is reduced by an increase in the threshold potential, rather than a decrease in slope of phase 4 depolarization.
· Propafenone considerably depresses impulse transmission and has profound effect on His- Purkinje as well as accessory pathway conduction by blocking Na+ channel.
Pharmacokinetics
· Flecainide is absorbed orally and is metabolized by CYP2D6 to multiple metabolites.
· Eliminate through urine.
· Propafenone is absorbed orally and undergoes variable first pass metabolism
· Propafenone is metabolized to active metabolites primarily via CYP2D6, and also by CYP1A2 and CYP3A4.
· The metabolites are excreted in the urine and the feces.
Adverse effect
· Flecainide and propafenone has some same adverse effect like Nausea, dizziness, blurred vision. But propafenone may also cause bronchospasm due to its β-blocking effects.
· It should be avoided in patients with asthma.
· Propafenone is also an inhibitor of P-glycoprotein.
· Both drugs should be used with caution with potent inhibitors of CYP2D6.
Dose
· Propafenone: 150 mg BD–300 mg TDS
· Flecainide: 50mg, dose not exceeding 300mg/day.
Therapeutic uses
· Flecainide is useful in the maintenance of sinus rhythm in atrial flutter or fibrillation in patients without structural heart disease (left ventricular hypertrophy, heart failure, atherosclerotic heart disease) and in treating refractory ventricular arrhythmias.
· Flecainide has a negative inotropic effect and can aggravate chronic heart failure.
· Propafenone is used for prophylaxis and treatment of ventricular arrhythmias, reentrant tachycardia involving AV node/accessory pathway and to maintain sinus rhythm in AF.
Class II (β-blocker)
· These drugs diminish phase 4 depolarization and, thus, depress automaticity, prolong AV conduction, and decrease heart rate and contractility.
· Propranolol has direct membrane stabilizing action at high doses.
· Propranolol has only mild depressant action on SA node automaticity, but marked decrease in the slope of phase-4 depolarization and automaticity occurs in SA node.
· Prolong the ERP of A-V node.
· Used for atrial flutter and fibrillation and for AV nodal reentrant tachycardia.
· In addition, β-blockers prevent life-threatening ventricular arrhythmias.
· Metoprolol is widely used in the treatment of cardiac arrhythmias. it reduces the risk of bronchospasm.
· It is extensively metabolized in the liver primarily by
· Esmolol is a very short acting β-blocker used for intravenous administration in acute arrhythmias that occur during surgery or emergency situations.
· It has a fast onset of action and a short half-life.
· Esmolol is rapidly metabolized by esterases in red blood cells.
Class III (Agents widening AP)
· Class III agents block potassium channels and, thus, diminish the outward potassium current during repolarization of cardiac cells.
· These agents prolong the duration of the action potential without altering phase 0 of depolarization or the resting membrane potential.
· Prolonged ERP.

(Prolong phase 3 repolarization without altering phase “0”)
Amiodarone
· Amiodarone is structurally related to thyroxine, also contain iodine.
· Amiodarone shows multiple action like, prolong APD and Q-T interval, block K+ channels, and partially block Na+ channels, more effective in depressing conduction in cells that are partially depolarized or have longer APD.
· Partially inhibit myocardial Ca2+ channels.
Therapeutic uses
· Severe refractory supraventricular and ventricular tachyarrhythmia.
· Atrial fibrillation or flutter.
Pharmacokinetics
· Amiodarone is incompletely absorbed after oral administration.
· Prolonged half-life of several weeks.
· Distributes extensively in adipose tissue.
· metabolized in liver mainly by CYP3A4
· t½ 3–8 weeks
Dose
· Amiodarone is mainly used orally 400–600 mg/day for few weeks, followed by 100–200 mg OD for maintenance therapy. 100–300 mg (5 mg/kg) slow i.v. injection over 30–60 min.
Adverse effect
· Pulmonary fibrosis
· Neuropathy
· Hepatotoxicity
· Corneal deposits
· Optic neuritis
· Blue-gray skin discoloration
· Hypo or hyperthyroidism
Interaction
· Amiodarone can increase digoxin and warfarin levels by reducing their renal clearance.
· Inducers and inhibitors of CYP3A4 respectively decrease and increase amiodarone levels.
Dronedarone
· Newly formulated non-iodinated congener of amiodarone, less toxic, but also less effective class III antiarrhythmic.
· Dronedarone is a multichannel blocker, inhibits delayed rectifier and other types of cardiac K+ channels, inward Na+ channel and L-type Ca2+ channel.
· Marked noncompetitive β adrenergic blocking activity.
· It increases myocardial APD, ERP and slows A-V conduction.
· Dronedarone is less lipophilic and is metabolized by CYP3A4 and CYP2D6; inhibitors of these iso-enzymes (Ketoconazole, erythromycin, metoprolol, etc.) markedly increase its blood levels.
· The elimination t½ is 24 hours.
· Side effects are mainly gastrointestinal disturbances, bradycardia, weakness, cough and dermatological reactions.
· Though it prolongs Q-T interval, risk of torsades de pointes is very low. Hypothyroidism, pulmonary fibrosis and peripheral neuropathy does not occur.
· Dronedarone is contraindicated in moderate-to-severe CHF, 2nd/3rd degree A-V block and in permanent AF.
Dose
· 400 mg BD oral
Dofetilide
Dofetilide is a pure potassium channel blocker. It can be used as a first-line antiarrhythmic agent in patients with persistent atrial fibrillation and heart failure or in those with coronary artery disease. Because of the risk of pro-arrhythmia, dofetilide initiation is limited to the inpatient setting. The half-life of this oral drug is 10 hours. The drug is mainly excreted unchanged in the urine. Drugs that inhibit active tubular secretion are contraindicated.
Ibutilide
Ibutilide is a potassium channel blocker that also activates the inward sodium current (mixed class III and IA action). Ibutilide is the drug of choice for chemical conversion of atrial flutter, but electrical cardioversion has supplanted its use. Ibutilide undergoes extensive first-pass metabolism and is not used orally. Because of the risk of QT prolongation and pro-arrhythmia, ibutilide initiation is limited to the inpatient setting.
Class IV (calcium channel blockers)

(Slow phase IV depolarization)
Verapamil
· Blocks the calcium channels and delay their recovery.
· It suppresses automaticity and reentry dependent on slow channel response.
· Prolongation of A-V nodal ERP.
· Verapamil has negative inotropic action due to interference with Ca2+ mediated excitation-contraction coupling in myocardium.
Uses
· PSVT; 5 mg i.v. over 2–3 min is effective in ~ 80% cases.
· For preventing recurrences of PSVT 60 to 120 mg TDS may be given orally.
· To control ventricular rate in AF or AFl.
Diltiazem
· Similar to verapamil but, bradycardia and depression of cardiac contractility are less marked.
· It is an alternative to verapamil for termination as well as prophylaxis of PSVT.
· Dose of diltiazem is 30, 60, 90 mg tabs, 25 mg/5 ml inj.
Digoxin
· Digoxin inhibits the Na+/K+-ATPase pump, ultimately shortening the refractory period in atrial and ventricular myocardial cells while prolonging the effective refractory period and diminishing conduction velocity in the AV node.
· Digoxin is used to control ventricular response rate in atrial fibrillation and flutter.
Adenosine
Adenosine is a naturally occurring nucleoside, but at high doses, the drug decreases conduction velocity, prolongs the refractory period, and decreases automaticity in the AV node. Intravenous adenosine is the drug of choice for abolishing acute supraventricular tachycardia. It has low toxicity but causes flushing, chest pain, and hypotension. Adenosine has an extremely short duration of action (approximately 10 to 15 seconds) due to rapid uptake by erythrocytes and endothelial cells.
Magnesium sulfate
Magnesium is necessary for the transport of sodium, calcium, and potassium across cell membranes. It slows the rate of SA node impulse formation and prolongs conduction time along the myocardial tissue. Intravenous magnesium sulfate is the salt used to treat arrhythmias, as oral magnesium is not effective in the setting of arrhythmia. Most notably, magnesium is the drug of choice for treating the potentially fatal arrhythmia torsades de pointes and digoxin-induced arrhythmias.

Hi….!! My name is Smrutiranjan Dash, From Odisha, India. Professionally I am Assistant Professor at The Pharmaceutical College, Barpali, Odisha, department of Pharmacology.

Phentermine is an appetite stimulant that suppresses the feeling of hunger and reduces the food intake on a normal basis.
This drug stimulates the central nervous system, which helps to improve attention and focus in individuals suffering from ADHD (attention-deficit hyperactivity disorder).
When it comes to Attention Deficit Hyperactivity Disorder the Adderall 30mg Generic Pills dosages are recommended.