BIOASSAY
· Bio-living, assay-test
· It is the process of determining the potency of the drug by using suitable biological system like animals, tissues, microbes etc.
PRINCIPLE OF BIOASSAY
· To compare the test substance with the International Standard preparation (IP, BP, USP) of the same.
· To find out how much test substance is required to produce the same biological effect, as produced by the standard.
APPLICATION
· To determine the potency of a drug.
· To determine the dose of a drug required to produced a therapeutic or toxic response (ED50, LD50).
· This process is used in the development of new molecules or preparation.
CHARACTERISTICS OF A GOOD ASSAY METHOD
· Sensitivity
· Specificity
· Repeatability
· Reproducibility
· Precision
· Accuracy
· Stability – tissue has to stay “bioassay-fit
BIOASSAY CAN BE PERFORMED ON
1. In-vivo → Intact animal
2. In-vitro → isolated tissues, specific cells, organisms.
· WHOLE ANIMALS
o Nor Adrenaline – Spinal Cat
o Cardiac Glycosides – Guinea Pig
o Insulin – Mice
o Estrogens – Ovariectamised Female Rat
· MICRO ORGANISMS
o Vit B12 – Euglena gracilis
o Tetracycline – Bacillus pumilus
· ISOLATED TISSUE
o Acetyl Choline – Frog Rectus Abdominus muscle
o Histamine – Guinea Pig ileum
o Adrenaline – Rat uterus
o Oxytocin – Rat uterus oestrogen primed
· DISPERSED CELLS
o Plasma LH estimation by stimulation of testosterone synthesis – on isolated Leydig cells
TYPES OF BIOASSAY
· There are mainly two types of bioassay;
1. Quantal assay
§ Direct end point assay (DEPA)
§ LD50 determination
2. Graded assay
§ Matching
§ Bracketing
§ Interpolation
§ Multiple point
→ Three point
→ Four point
→ Six point
§ Cumulative dose response
QUANTAL ASSAY
· The response is in the form of “all or none”, i.e. either no response or maximum response. Drugs producing quantal effect can be bioassayed by end point method.
· The threshold dose producing a predetermined effect is measured
· Comparison between the results of standard and the test
· E.g: Bioassay of digitalis in cats, Insulin induced hypoglycemic convulsions in rat.

GRADED ASSAY
· Response is proportional to the dose and response may lie between no response and the maximum response.

MATCHING OR BRACKETING
· A constant dose of the standard is bracketed by varying dose of test sample.
· until an exact matching between the response of standard & that of the test sample is matched.
· Strength of unknown/test drug can be found by simple interpolation of bracketed response.
ADVANTAGES
· Simple & Faster method.
· Amount of test drug available is small
· Does not involve complicated calculations
· Does not depend on DRC
DISADVANTAGES
· less accurate
· Time consuming
· Cannot get exact match of response
· Quantitative difference between test & standard not obtained.
INTERPOLATION ASSAY
· The concentration of test drug is interpolated form the dose response curve graph (DRC).
· The DRC of standard drug is plotted first, then the different concentrations of the test drug are plotted.
· The dose of the test drug which comes at the linear log dose-response relationship is interpolated from the dose response plot.

MULTIPLE POINT ASSAY
· The responses are taken several times and mean of each is taken. So that the chances of errors are minimized.
· These assays are performed by the selection of 1 or more dose responses of test compound and these responses are compared with 2 or more responses of standards.
THREE-POINT BIOASSAY
· In this bioassay total three responses selected from DRC for standard as well as test.
· One for test and two standard responses are selected from DRC.
· This method depends on the latin square randomization of three responses from DRC.
· The response selection is made between 25-75%.
· Estimates of relative potency are then obtained as the displacement of parallel log dose response lines of standard and test compound.

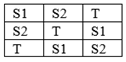
Latin square randomization



FOUR-POINT BIOASSAY
· Four-point bioassay implies 2 responses of standard and 2 of test from the DRC for the consecutive 16 response of Latin square randomization.

Latin square randomization



SIX-POINT BIOASSAY
· The responses obtained for the 6-point is ‘36’ and ‘64’ for 8-point.
· Less adopted because of time consuming, but it reduces error.
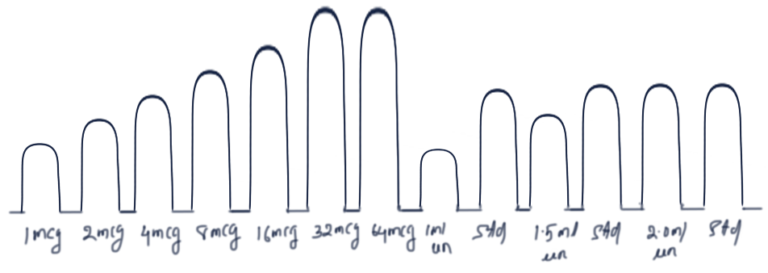
CUMULATIVE DOSE RESPONSE CURVE
· Increase concentration of drug in bath fluid step by step without washing out the preceding doses.
· Continue till super-maximal effect is seen.
· Dose response curve is plotted.

Hi…!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
