General principle of chemotherapy
Chemotherapy
· Chemo – Chemical, Therapy – Treatment
· The treatment of disease by means of chemicals that have a specific toxic effect upon the disease producing microorganisms or that selectively destroy cancerous tissue.
Antibiotics
· Antibiotics are the substance produce by microorganism, which selectively suppress the growth or kill other microorganism at very low concentration.
Anti-microbial (Chemotherapeutic agents + Antibiotics)
· Any substance of natural, synthetic, semisynthetic origin which at low concentrations kill or inhibit the growth of micro-organisms but causes little or no damage of host.
History of chemotherapy
· Father of chemotherapy – Paul Ehrlich – 1908 (Arsphenamine)
· Penicillin – Alexander Fleming
· Gerhard Domagk – Sulfonamide – 1932
Principle of Anti-microbial therapy
1. Diagnosis
· Site of infection
· Organism
· Drugs (Sensitive)
2. Treatment (Whether chemotherapy is necessary or not)
· Acute infection – Require chemotherapy
· Chronic infection – Poorly respond (Surgery necessary)
3. Selection of drugs
· Activity of antimicrobial activity.
· Pharmacokinetic factor
· Patient related factor (Allergy, renal disease)
4. Frequency and duration of drug administration
· Optimize dose should be used for therapy.
· Inadequate dose may develops resistance.
5. Continue therapy
· Some infection treated within 5-10 days, but some infection need more time to cure.
e.g. Typhoid fever
Tuberculosis
Infective endocarditis (Inflammation of heart valve)
6. Prophylactic chemotherapy
· To avoid surgical site infection
Classification of antimicrobial
A. Chemical structure
B. Mechanism of action
C. Types of organism
D. Spectrum of activity
E. Types of action (Bacteriostatic and bactericidal)
F. Source of antibiotic
A. Chemical structure
1) Sulfonamides and related drugs:
Dapsone (DDS), Sulfadiazine, Paraaminosalicylic acid (PAS)
2) Diaminopyrimidines:
Trimethoprim, Pyrimethamine
3) Quinolones:
Nalidixic acid, Norfloxacin, Ciprofloxacin
4) Beta lactam antibiotics:
Penicillins, Cephalosporins
5) Tetracyclines:
Oxytetracycline, Doxycycline
6) Nitrobenzene derivative:
Chloramphenicol
7) Aminoglycosides:
Streptomycin, Gentamycin, Amikacin, Neomycin
8) Macrolides antibiotics:
Erythromycin, Clanthromycin, Azithromycin
9) Lincosamide antibiotics:
Clindamycin
10) Glycopeptide antibiotics:
Vancomycin
11) Oxazolidinone
Linezolid
12) Polypeptide antibiotics:
Polymyxin-B, Bacitracin, Tyrothricin
13) Nitrofuran derivatives:
Nitrofurantoin
14) Nitroimidazoles:
Metronidazole, Tinidazole
15) Nicotinic acid derivatives:
Isoniazid, Pyrazinamide, Ethionamide
16) Polyene antibiotics:
Amphotericin-B, Nystatin, Hamycin
17) Azole derivatives:
Miconazole, Clotrimazole, Ketoconazole, Fluconazole
18) Others:
Rifampin, Ethambutol, Griseofulvin
B. Mechanism of action
1) Inhibition of cell wall synthesis
e.g. Penicillins, Cephalosporins, Cycloserine, Vancomycin, Bacitracin.
2) Inhibition of protein synthesis
e.g. Tetracyclines, Chloramphenicol, Erythromycin, Clindamycin, Linezolid.
3) Injury from cell membrane
e.g. Polymyxin-B
4) Inhibition of synthesis of essential metabolites
e.g. Sulfonamide, Sulfone, PAS, Trimethoprim, Pyrimethamine, Metronidazole
5) Inhibition of Nucleic acid replication and transcription
e.g. Rifampin. Quinolones.
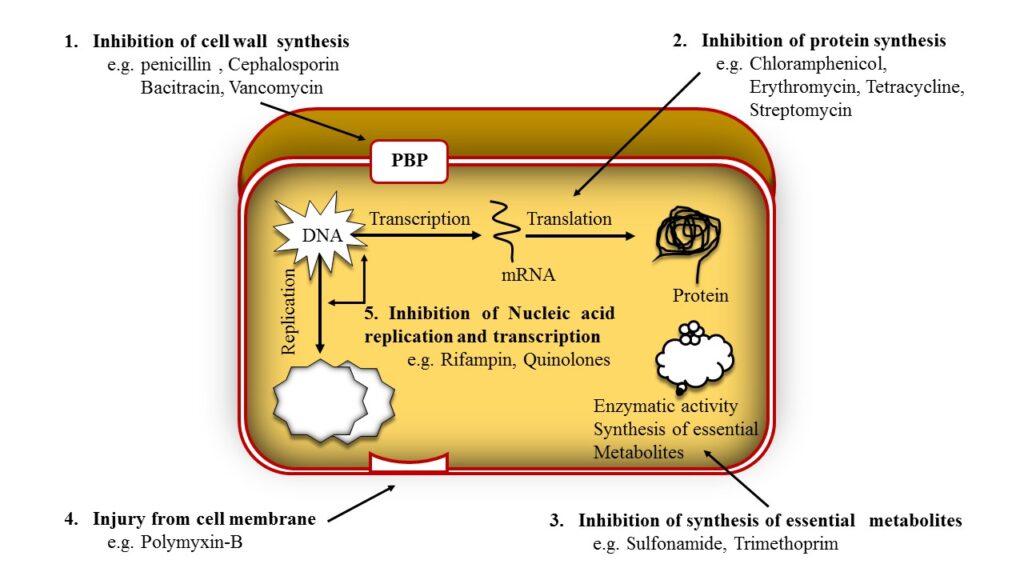
Inhibition of cell wall synthesis
· The penicillin interfere with last step of bacterial cell wall synthesis (transpeptidastion or cross linkage).
· Resulting in exposure of the osmotically less stable membrane.
· Cell lysis can occur, either through osmotic pressure or through autolysins.
1. Penicillin Binding Protein (PBP)
· The PBPs are the bacterial enzymes involved in the synthesis of the cell wall and in the maintenance of the morphologic feature of bacterium.
· Antibiotics interfere to the cell wall synthesis and also lead to structural changes or lysis of susceptible bacteria
2. Inhibition of transpeptidase
· Penicillin inhibits transpeptidase – catalyzed reaction, difficulty in the formation of cross-links essential for cell wall integrity. As a result blockade of cell wall synthesis.
3. Production of autolysin
· Many bacteria, particularly the gram positive cocci, produce degradative enzyme (Autolysins) that participate in normal remodeling of the bacterial cell wall synthesis.
· In the presence of penicillin, the degradative action of autolysin proceed in the absence of cell wall synthesis.
· Thus antibacterial effect on penicillin is the result of both inhibition of cell wall synthesis and destruction of existing cell wall by autolysins.
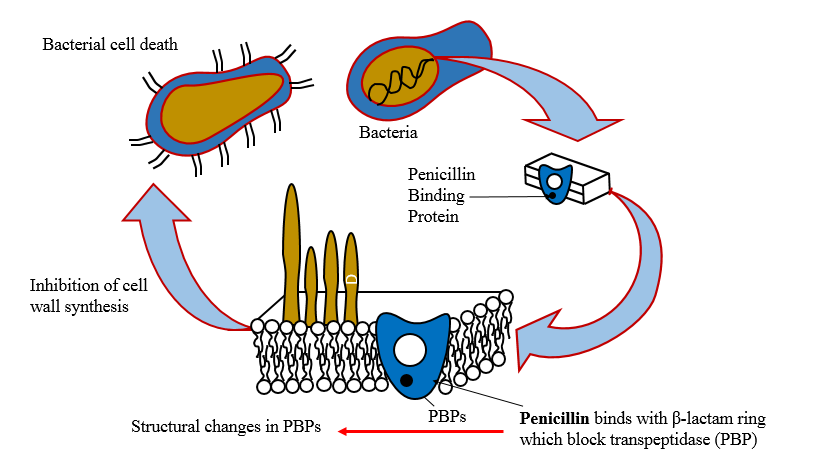
Inhibition of protein synthesis
· Entry of the agents into susceptible organism is mediated both by passive diffusion and by an energy-dependent transport protein mechanism to the bacterial cytoplasmic membrane.
· The drugs binds reversibly to the 30s, 50s subunit etc. of the bacterial ribosome, thereby blocking access of t-RNA to mRNA ribosome complex.
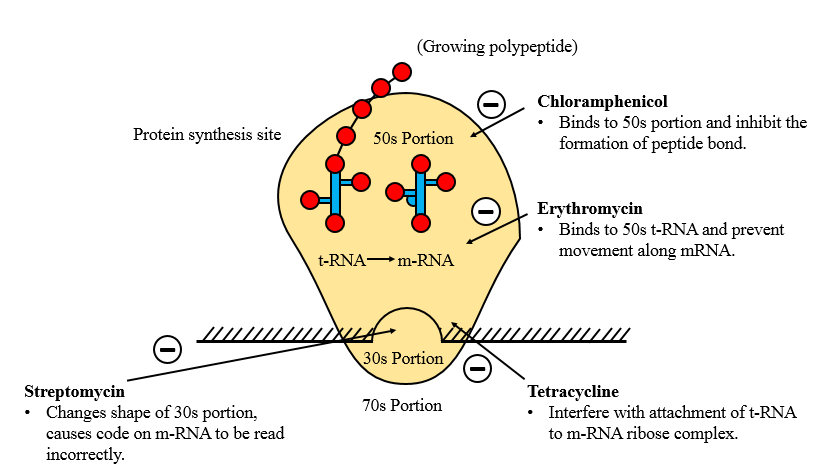
Inhibition of Nucleic acid synthesis
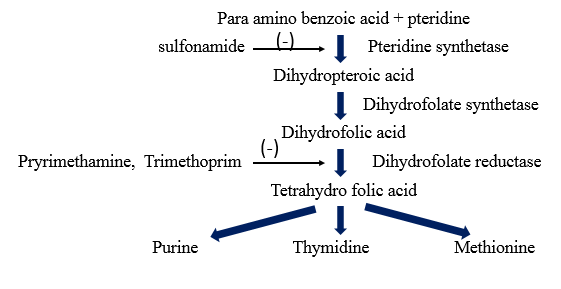
ü To interfere with nucleic acid synthesis in five different steps.
- By inhibiting the synthesis of the nucleotide.
• This can be accomplished by an effect on the metabolic pathways that generate nucleotide precursor. Example Folate.
- By altering the base-paring properties of DNA templet.
• Agents that intercalate (bilateral) in the DNA have this effect. Example: Proflavin, Acriflavin.
- By inhibiting either DNA or RNA polymerase.
• Rifampicin inhibit the synthesis of bacteria DNA gyrase or RNA polymerase (Prokaryotic not Eukaryotic).
4. By inhibiting DNA gyrase, which uncoils supercoiled DNA to allow transcription.
• The flroquinolones (Ciprofloxacin, cinoxacin, naldixic acid and norfloxacin) act by inhibiting DNA gyrase, and this chemotherapeutic agents are particularly useful for treating infections with gram negative organisms.
5. By direct effect on DNA itself.
Inhibition of folic acid synthesis (Bacteriostatic)
A. Types of organism
Antibacterial
o Penicillin, Aminoglycoside, Erythromycin
Antiviral
o Acyclovir, Amantadine, Zidovudine
Antifungal
o Griseofulvin, Amphotericin-B, Ketoconazole
Anti-protozoal
o Chloroquine, Pyrimethamine, Metronidazole
Anthelmintic
o Mebendazole, niclosamide, Diethyl Carbamazine
B. Spectrum of activity
Narrow spectrum
• Penicillin-G
• Streptomycin
• Erythromycin
(Effective against either gram +ve or gram -ve)
Broad spectrum
• Tetracycline
• Chloramphenicol
(Effective against a wide range of bacteria, both gram +ve and gram -ve)
C. Side of action
Bacteriostatic
• Inhibition the growth of bacteria.
• Sulfonamide, Tetracycline, Chloramphenicol, Erythromycin, Ethambutol
Bactericidal
• Kill the microorganisms
• Penicillin, Aminoglycosides, Polypeptides, Rifampin, Isoniazid, Vancomycin, Ciprofloxacin, Metronidazole, Cotrimazole.
D. Sources of antibiotics
Fungi
• Penicillin, Griseofulvin, Cephalosporin
Bacteria
• Polymyxin-B, Tyrothricin, Aztreonam, Bacitracin
Actinomycetes
• Aminoglycosides, Macrolides, Tetracycline, Polyenes, Chloramphenicol
Penicillin
• Penicillin was the first antibiotic to be used clinically in 1941.
• It is originated from fungus Penicillium notatum.
• Recent source of penicillin Penicillium chrysogeum.
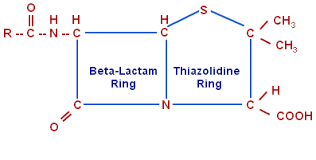
CLASSIFICATION
1. Acid-resistant alternative to penicillin G
Phenoxymethyl penicillin (Penicillin V).
2. Penicillinase-resistant penicillins
Methicillin, Cloxacillin, Dicloxacillin.
3. Extended spectrum penicillins
(a) Aminopenicillins:
Ampicillin, Bacampicillin, Amoxicillin.
(b) Carboxypenicillins:
Carbenicillin.
(c) Ureidopenicillins:
Piperacillin, Mezlocillin.
β-lactamase inhibitors
Clavulanic acid, Sulbactam, Tazobactam
Mechanism of action
• All beta lactam antibiotic interfere with the synthesis of bacterial cell wall.
• Generally the bacteria synthesize UDP-N acetylmuramic acid pentapeptide (Park Nucleotide) and UDP-N-acetyl glucosamine.
• The final step is cleavage of the terminal D-alanine of the peptide chains by transpeptidases, so that cross linking between the peptide chains occur.
• Penicillin inhibit the transpeptidases so that cross linking does not takes place.
• When the susceptible bacteria divide in the presence of a beta lactam antibiotic then the cell wall deficient forms are produced.
• Because the interior of the bacterium is hyperosmotic, the cell wall deficient (CWD) forms swell and burst leads to bacterial lysis.
Bacterial resistance
• Some bacteria does not response PnG because the enzyme and PBPs are located deeper under lipoprotein barrier.
• Hence PnG is unable to penetrate or less affinity.
• The primary mechanism of acquired resistance is production of penicillinase.
Penicillinase
• Penicillinase It is a narrow spectrum β-lactamase which opens the β-lactam ring and inactivates PnG and some closely related congeners.
• Staphylococci and some strains of gonococci, B. subtilis, E. coli, H. influenza and few other bacteria produce penicillinase.
• The gram-positive penicillinase produces large quantities of the enzyme which diffuses into the surroundings and can protect other inherently sensitive bacteria. While gram negative bacteria it is found in less extend.
Pharmacokinetics Penicillin G
• Penicillin-G destroyed by gastric acid.
• Rapidly absorbed from i.m.
• Plasma peak level 30min.
• Poorly penetrate CSF.
• About 60% plasma protein bound.
• Less metabolism due to rapid excretion.
• Plasm t1/2 30min (Adult).
Preparation and doses
• Sod. Penicillin G (crystalline form) 0.5-5 MU
Repository penicillin G injection
• Insoluble salts of penicillin G: given deep i.m. (never i.v.).
- Procain penicillin G injection
• 0.5-1 MU i.m. (suspension) 12-24 hourly.
• Plasma concentration low but sustain for 12-24 hours.
- Fortified Procain penicillin G injection
• 3 lac U Procain penicillin G
- Benzanthin penicillin
• 0.6-2.4 MU, every 2-4 weeks
Adverse effect
• Penicillin is a safer drug (nontoxic) up to 20 MU has been injected in a day without any organ toxicity.
1) Local irritant and direct toxicity
• Pain at i.m. injection site
• Nausea
• Thrombophlebitis (an inflammation process that causes blood clot to form block vein).
• Toxicity to brain (mental confusion, muscular twitching, convulsion and coma).
• Penicillin G not recommended for intrathecal injection (Arachnoiditis).
2) Hypersensitivity
• Rash, itching, urticarial and fever.
• Wheezing
• Angioneurotic edema (painless swelling under the skin)
• Serum sickness (allergic reaction caused by immune response)
• Exfoliative dermatitis (redness and peeling of the skin)
• Anaphylaxis (rare)
Super infection
• Rare due to narrow sputum.
Jarisch – Herxheimer reaction
• If penicillin injected in a syphilitic patient may produce fever, myalgia (pain in muscle), exacerbation of lesion, vascular collaps.
Uses
ü Streptococcal infection
• Pharyngitis
• Otitis media
• Scarlet fever
• Rheumatic fever
ü Pneumococcal infection
• Pneumonia
• Meningitis
• 3-6 MU (every 6 hour DOC)
ü Meningococcal infection
• Meningitis (high dose i.v.)
ü Gonorrhoea
• Resistant to penicillin
ü Syphilis
• DOC 1.2 MU of procaine penicillin for 10 days or with 1-3 weekly doses of 2.4 MU Benzanthin penicillin
ü Diphtheria (-2 MU daily for 10 days).
ü Tetanus (6-12 MU/day).
ü Anthrax, actinomycosis, rat bite fever (DOC).
ü Prophylactic uses
• Rheumatic fever
• Bacterial endocarditis
• Agranulocytosis patient (lower WBCs)

Hi…!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
About The Author
Hi...!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
