Protein and Amino acid
· The term protein is derived from the Greek word proteios, meaning holding the first place.
· Proteins are the most abundant organic molecules of the living system.
· They occur in every part of the cell and constitute about 50% of the cellular dry weight.
· Proteins form the fundamental basis of the structure and function of life.
Function of protein
· Protein is the major source of energy.
· Protein plays a vital role in the maintenance of body tissue, development, and repair.
· Protein produces enzymes that increase the rate of chemical reactions in the body.
· Protein is involved in some hormonal function that helps to regulate body function and growth.
Elemental composition of proteins
· Carbon : 50 – 55%
· Hydrogen : 6 – 7.3%
· Oxygen : 19 – 24%
· Nitrogen : 13 – 19%
· Sulfur : 0 – 4%
· Protein may also contain P, Fe, Cu, I, Mg, Mn, Zn, etc.
· The content of Nitrogen is the essential component of the proteins, on average is 16%.
· Estimation of nitrogen in the laboratory is used to find out the amount of protein in biological fluids and foods.
Classification of protein
1. Simple protein
· Those which give one amino acid only upon hydrolysis.
· Example: Albumins, Globulins, Glutelins, Histone, Scleroproteins
2. Conjugated protein
· Those which give an amino acid and a non-protein group upon hydrolysis.
· Example: Nucleoproteins, Mucoprotein, Glycoproteins, Phosphoproteins,
3. Derived protein
· Those are derived from simple and conjugated proteins.
1. Simple protein
a) Albumins:
o Globular protein is insoluble in water and dilutes salt solution.
o Precipitated by saturation with (NH4)2SO4 solution.
o Coagulated by heat.
o Found in plant and animal tissue.
o Example: Blood (Serumbumin)
o Milk (Lactalbumin)
o Egg white (Ovolbumin) etc.
b) Globulins:
o Globular Protein is sparingly soluble in water and neutral solutions.
o Precipitated by dilute Ammonium Sulphate.
o Coagulated by Heat.
o Distributed in both plant and animal tissues.
o Example: Blood (Serum Globulins)
o Muscle (Myosin)
o Potato (Tuberin) etc.
c) Glutelins
o Insoluble in water and dilute salt solutions.
o Soluble in dilute acids.
o Found in grains & cereals.
o Example: wheat (Glutenin)
o Rice (Oryzenin) etc.
d) Histones
o Soluble in water, salt solutions & dilute acids.
o Insoluble in Ammonium Hydroxide.
o Yields a large amount of Lysine & arginine.
o Combined with nucleic acids within cells.
e) Scleroproteins
o Connective tissues and hard tissues.
o Fibrous protein is insoluble in all solvents.
o Resistant to digestion.
2. Conjugated protein
a) Nucleoproteins
o It contains nucleic acids, nitrogen, and phosphorus.
o It is present in chromosomes and all living forms as a combination of protein with either DNA or RNA.
b) Mucoprotein
o Saliva (Mucin) and Egg white (Ovomucoid).
o Proteins are combined with amino sugars, sugar acids, and sulfates.
c) Glycoproteins
o Bones (Osseomucoid), Tendons (Tendomucoid), and Cartilage (Chondromucoid).
o Containing more than 4% Hexosamine, mucoproteins; if less than 4%, then Glycoproteins.
d) Phosphoproteins
o Milk (Casein) and Egg yolk (Ovovitellin).
o Phosphoric acid joined in ester linkage to protein.
3. Derived Proteins
a) Proteans
o Edestan (from Elastin) and Myosin.
o It results from the short action of acids or enzymes
o Insoluble in water.
b) Proteases
o The intermediate product of protein digestion
o Soluble in water.
o Not coagulated by heat.
o Precipitated by saturated ammonium sulfate
o Result from partial digestion of protein by pepsin or trypsin.
c) Peptones
o The intermediate product of protein digestion.
o Same properties as proteases except that they cannot be salted out.
o Smaller molecular weight than proteases.
d) Peptides
o The intermediate product of protein digestion.
o Two or more amino acids are joined by a peptide linkage.
o Hydrolyzed to individual amino acids.
o
Proteins are polymers of amino acids
· All proteins have common properties i.e. on hydrolysis of protein with HCl for several hours yield L-α-amino acid.
· Therefore proteins are the polymers of L-α-amino acids.
Amino acid
· 300 amino acids are present but 20 amino acids are known as standard amino acids are repetitively found in the structure of proteins.
· Amino acids are a group of organic compounds containing two functional groups amino and carboxy.
· The amino group (—NH2) is basic while the carboxyl group (—COOH) is acidic.
General structure of amino acid
· The amino acids are termed D-amino acids if both the carboxyl and amino groups are attached to the same carbon atom.
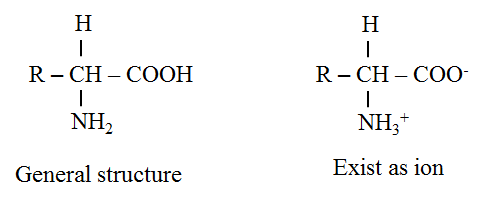
· The D-carbon atom binds to a side chain represented by R which is different for each of the 20 amino acids found in proteins.
· The amino acids mostly exist in the ionized form in the biological system.
Optical isomers of amino acids
· The amino acid (except glycine) have optical isomers (possesses four distinct groups i.e. R, H, COO–, NH3+).
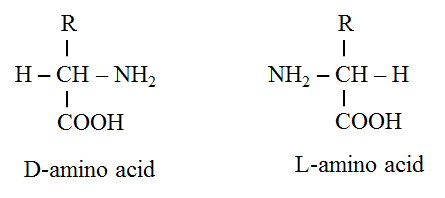
Classification of amino acids
· Based on
o Structure
o Chemical nature
o Nutritional requirement
o Metabolic fate
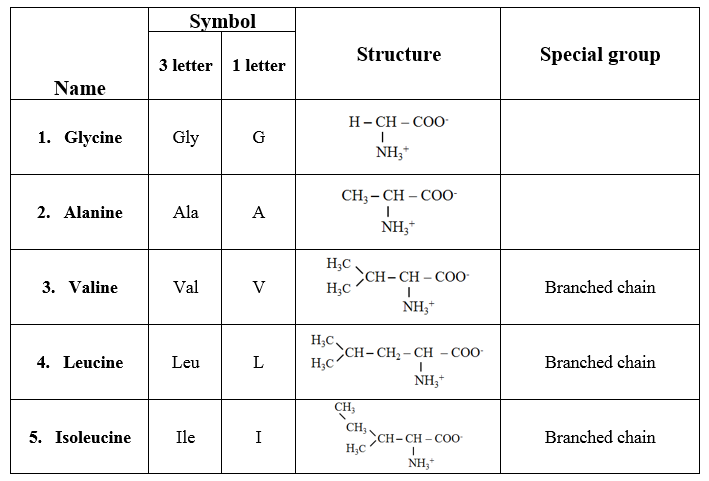
Amino acid classification based on the structure
1. Amino acids with aliphatic side chains
· These are monoamino monocarboxylic acids.
· Some amino acids contain branched aliphatic side chains, hence they are referred to as branched-chain amino acids.
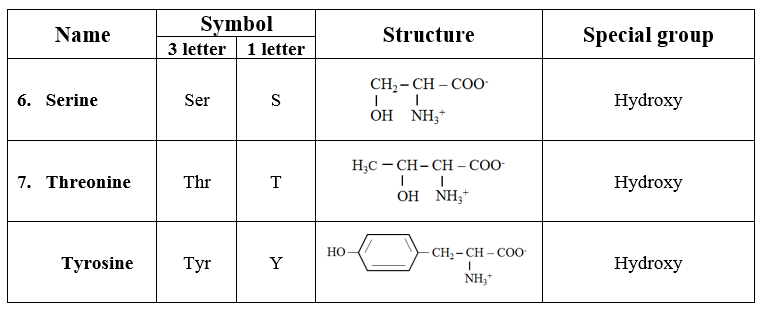
2. Hydroxyl group-containing amino acids
· Containing hydroxyl group.
3. Sulfur-containing amino acids
· Cysteine with sulfhydryl group and methionine with thioether group are the two amino acids incorporated during protein synthesis.
· Cystine is formed by the condensation of two molecules of cysteine.
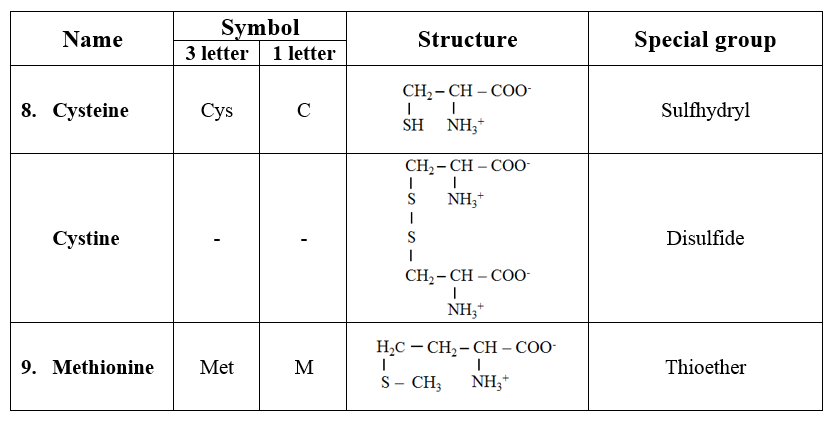
4. Acidic amino acids and their amides
· Aspartic acid and glutamic acids are dicarboxylic monoamino acids while asparagine and glutamine are their respective amide derivatives.
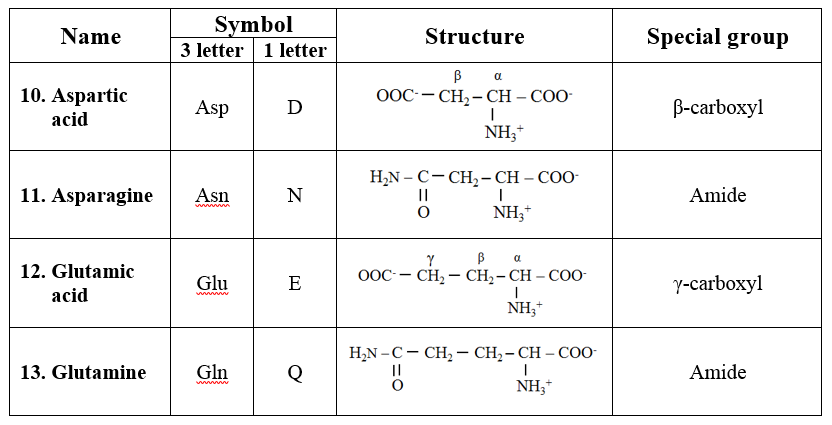
5. Basic amino acids
· They are dibasic monocarboxylic acids. They are highly basic in character.
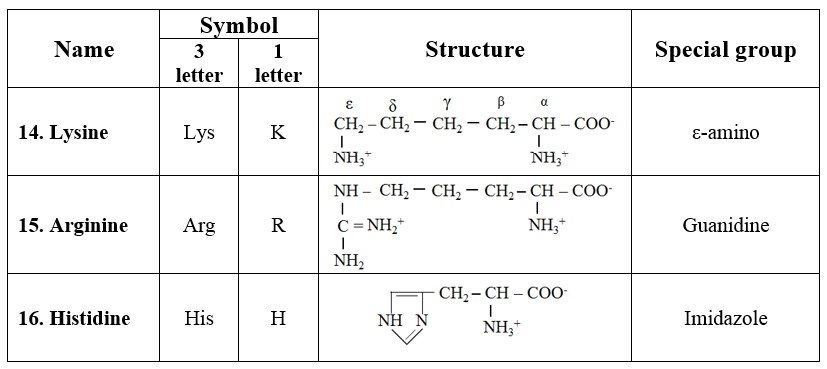
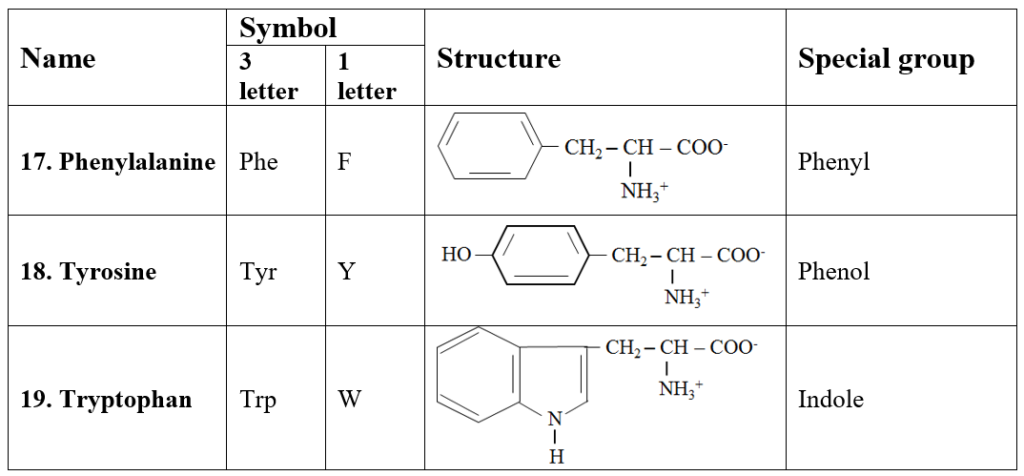
6. Aromatic amino acids
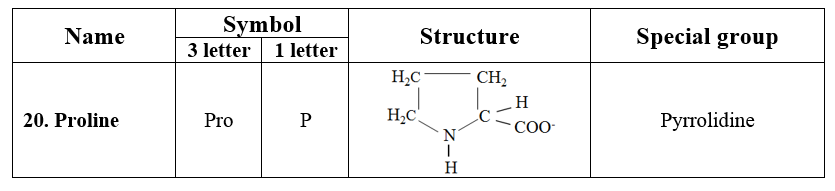
7. Imino acids
· Proline containing pyrrolidine ring is a unique amino acid. It has an amino group (NH), instead of an amino group (NH2) found in other amino acids. Therefore, proline is a D-imino acid.
Classification based on chemical nature
Amino acid-based on polarity
1. Non-polar amino acid
o These types of amino acids are referred to as hydrophobic.
o They have no charge on the ‘R’ group.
o Example:
§ Alanine
§ Leucine
§ Isoleucine
§ Valine
§ Methionine
§ Phenylalanine
§ Tryptophan
§ Proline
2. Polar amino acids with no charge on the ‘R’ group
o Carries no charge on the ‘R’ group.
o This amino acid possesses hydroxyl, sulfhydryl, and amide groups and participates in the hydrogen bonding of protein structure.
o Example:
§ Glycine (also consider in this category R = H)
§ Serine
§ Threonine
§ Cysteine
§ Glutamine
§ Asparagine and tyrosine
3. Polar amino acids with positive ‘R’ group
o Amino acids lysine, arginine, and histidine are included in this group.
4. Polar amino acids with negative ‘R’ group
o The dicarboxylic monoamino acids, aspartic acid, and glutamic acid are considered in this group.
Nutritional classification of amino acids
· The 20 amino acids are required for the synthesis of a variety of proteins.
· Some are produced by biological systems and others are derived from the diet.
· Based on nutritional requirement the amino acid is divided into two groups;
1. Essential amino acid
2. Non-essential amino acid
Essential amino acid
· The amino acids which cannot be synthesized by the body and, therefore, need to be supplied through the diet are called essential amino acids.
· In this amino acid, there are 10 amino acids are involved in the growth and maintenance of the body.
· Examples: A.V. HILL, MP., T. T
o Arginine
o Valine
o Histidine
o Isoleucine
o Leucine
o Lysine
o Methionine
o Phenylalanine
o Threonine
o Tryptophan
· Arginine and histidine cannot be synthesized by children and it is synthesized by adults, hence these are considered as semi-essential amino acids.
· Thus, 8 amino acids are essential while 2 are semi-essential.
Non-essential amino acid
· The body can synthesize about 10 amino acids to meet the biological needs, hence they need not be consumed in the diet.
· Examples:
o Glycine
o Alanine
o Serine
o Cysteine
o Aspartate
o Asparagine
o Glutamate
o Glutamine
o Tyrosine and Proline
Amino acid classification based on their metabolic fate
· The carbon skeleton of amino acids can serve as a precursor for the synthesis of glucose (glycogenic) or fat (ketogenic) or both.
· Based on metabolic fate, the amino acids are divided into three groups.
· 1. Glycogenic amino acid: These amino acids can serve as precursors for the formation of glucose or glycogen. e.g. alanine, aspartate, glycine, methionine, etc.
· 2. Ketogenic amino acids: Fat can be synthesized from these amino acids. Two amino acids leucine and lysine are exclusively ketogenic.
· 3. Glycogenic and ketogenic amino acids: The four amino acids isoleucine, phenylalanine, tryptophan, tyrosine are precursors for the synthesis of glucose as well as fat.
Properties of amino acid
A. Physical properties
1. Solubility: Most amino acids are usually soluble in water and insoluble in organic solvents.
2. Melting point: above 2000C.
3. Taste: Gly, Ala, Val (sweet), leu (tasteless), Arg, Ile (Bitter), MSG (monosodium glutamate) – used as a flavoring agent in the food industry. Increased consumption of MSG may cause Chinese restaurant syndrome (flu-like symptoms).
4. Optical properties:
· All the amino acids except glycine possess optical isomers due to the presence of asymmetric carbon atoms.
· Some amino acids also have a second asymmetric carbon e.g. isoleucine, threonine.
5. Amino acids as ampholytic:
· Amino acids contain both acidic (COOH) and basic (NH2) groups.
· They can donate a proton or accept a proton, hence amino acids are regarded as ampholytes.
· Zwitterion or dipolar ion:
o The term zwitterion is derived from German words mean hybrid. It contains both positive and negative ionic groups.
o In strongly acidic pH (low pH), the amino acid is positively charged (cation) while in strongly alkaline pH (high pH), it is negatively charged (anion)
o Leucine consists of pH 6.0, so it carries both positive and negative charges and exists as a zwitterion.
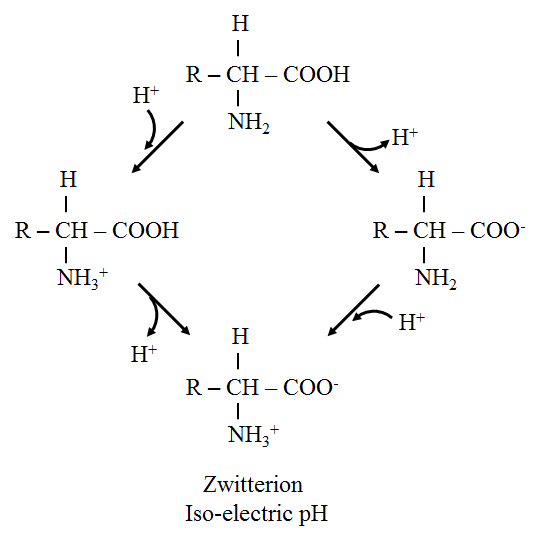
· Isoelectric point:
o It is defined as the pH at which a molecule exists as a zwitterion or dipolar ion and carries no net charge. Thus, the molecule is electrically neutral.
· Titration of amino acid:
o At low pH, leucine exists in a fully protonated form as a cation. As the titration proceeds with NaOH, leucine loses its protons, and an isoelectric pH (pI), becomes a zwitterion. Further titration results in the formation of an anionic form of leucine.
B. Chemical properties
Reactions due to COOH group
1. Amino acids form salts (COONa) with bases and esters (COORc) with alcohols.
2. Decarboxylation:
· Amino acids undergo decarboxylation to produce corresponding amines.
· This reaction assumes that formation of many biologically important amines in the living cells.
· These include histamine, tyramine and γ-amino butyric acid (GABA) from the amino acids histidine, tyrosine and glutamate, respectively.
3. Reaction with ammonia: The carboxyl group of dicarboxylic amino acids reacts with NH3 to form amide.
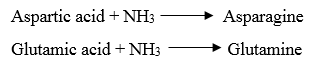
4. Reactions due to -NH2 group
· The amino groups behave as bases and combine with acids (e.g. HCl) to form salts (NH3 +Cl–).
5. Reaction with ninhydrin:
· The D-amino acids react with ninhydrin to form a purple, blue or pink colour complex (Ruhemann’s purple).
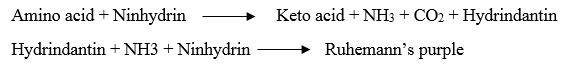
· Ninhydrin reaction is effectively used for the quantitative determination of amino acids and proteins.
6. Colour reactions of amino acids:
· Amino acids can be identified by specific colour reactions.
7. Transamination:
· Transfer of an amino group from an amino acid to a keto acid to form a new amino acid.
8. Oxidative deamination:
· The amino acids undergo oxidative deamination to liberate free ammonia.
Amino acids useful as drugs
· There a certain non-standard amino acids that are used as drugs.
· D-Penicillamine (D-dimethylglycine), a metabolite of penicillin, is employed in the chelation therapy of Wilson’s disease. This is possible since D-penicillamine can effectively chelate copper.
· N-Acetylcysteine is used in cystic fibrosis, and chronic renal insufficiency, as it can function as an antioxidant.
· Gabapentin (J-aminobutyrate linked to cyclohexane) is used as an anticonvulsant.
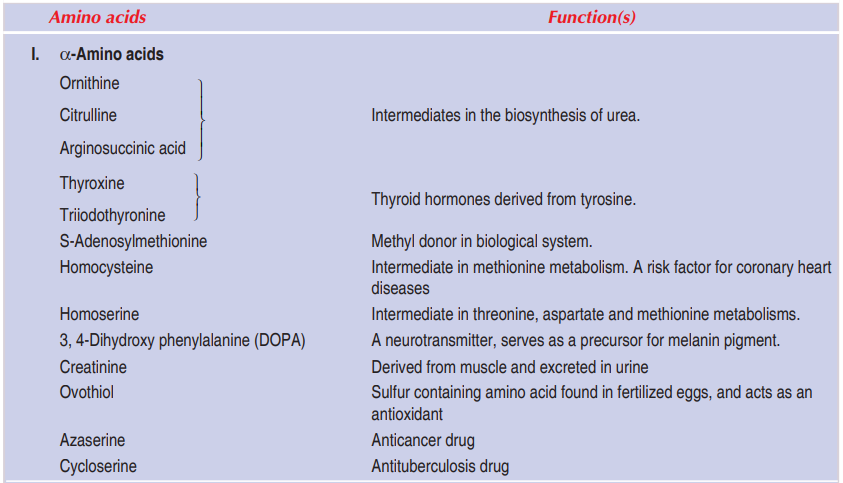
A selected list of important non-protein amino acids along with their functions

Structure of protein
1. Primary structure of protein
· The linear sequence of amino acids with protein called primary structure of protein.
· Each amino acid of protein determined by the genes contained in DNA.
· Abnormalities in the amino acid sequence shows that variety of genetic diseases.
Peptide bond
· The amino acids are held together in a protein by covalent peptide bonds or linkages.
· Formation of a peptide bond: When the amino group of an amino acid combines with the carboxyl group of another amino acid, a peptide bond is formed.
· Peptides containing more than 10 amino acids (decapeptide) are referred to as polypeptides.
Secondary structure
· The conformation of polypeptide chain by twisting or folding is referred to as secondary structure.
· The amino acids are located close to each other in their sequence.
· Two types of secondary structures, alpha-helix and beta-sheet, are mainly identified.
Alpha Helix
· Proposed by Pauling and Corey (1951).
· D-Helix is the most common spiral structure of protein.
· Tightly packed, coiled polypeptide back bone core.
· Side chain extended towards.
· Stabilized by H bonding between carbonyl oxygen amide hydrogen.
· Alpha helix segments are found many globular proteins like myoglobin, troponin-C.
Beta plated sheet
· Formed when two or more polypeptides line up side by side.
· Individual polypeptide is β-strand.
· Stabilized by hydrogen bond between amide and carbonyl groups of adjacent chains.
Tertiary structure
· The three-dimensional arrangement of protein structure is referred to as tertiary structure.
· It is a compact structure with hydrophobic side chains held interior while the hydrophilic groups are on the surface of the protein molecule.
· This type of arrangement ensures stability of the molecule.
· Bonds of tertiary structure: Besides the hydrogen bonds, disulfide bonds (-S -S), ionic interactions (electrostatic bonds), hydrophobic interactions and van der Waals forces also contribute to the tertiary structure of proteins.
· Domains: polypeptide chain containing more than, 200 residues of amino acid and it consist of two or more domains.
Quaternary structure
· The biological function of some protein molecules consist of two or more polypeptides which may be identical or unrelated.
· Such proteins are termed as oligomers and possess quaternary structure.
· The individual polypeptide chains are known as monomers, protomers or subunits.
· A dimer consist of two polypeptides while a tetramer has four.
· The sub units are held together by non-covalent bond namely hydrogen bonds, hydrophobic interactions and ionic bonds.
Properties of protein
1. Solubility
· Due to huge size of protein molecules Proteins form colloidal solutions instead of true solutions in water.
2. Molecular weight
· Molecular weight of protein is vary, due to it dependent on the number of amino acid residues.
· Each amino acid on an average a molecular weight of about 110. The majority of protein may be composed of 40 to 4,000 amino acids with a molecular weight ranging from 4,000 to 440,000.
· Example:
o Insulin-5,700
o Myoglobin-17,000
o Hemoglobin-64,450
o Serum albumin-69,000.
3. Shape
· Wide variety of shape.
· Example:
o Globular (insulin)
o Oval (albumin)
o Fibrous or elongated (fibrinogen)
4. Isoelectric pH
· The nature of the amino acids (particularly their ionizable groups) determines the pI of a protein.
· At isoelectric pH, the proteins exist as zwitterions or dipolar ions.
· They are minimum solubility, maximum precipitability and least buffering capacity.
· Example:
o Pepsin-1.1
o Casein-4.6
o Human albumin-4.7
o Urease-5.0
o Hemoglobin-6.7
o Lysozyme-11.0
5. Acidic and basic proteins
· Proteins in which the ratio (H Lys + H Arg) / (H Glu + H Asp) is greater than 1 are referred to as basic proteins. For acidic proteins, the ratio is less than 1.
6. Precipitation of proteins
· Proteins exist in colloidal solution due to hydration of polar groups (COO–, NH3 +, OH).
· Proteins can be precipitated by dehydration or neutralization of polar groups.
· Precipitation at pl:
o The proteins are least soluble at isoelectric pH. Certain proteins (e.g. casein) get easily precipitated when the pH is adjusted to pI (4.6 for casein).
o Example: Formation of curd from milk (slow precipitation). This occurs due to the lactic acid produced by fermentation of bacteria which lowers the pH to the pI of casein.
· Precipitation by salting out:
o The process of protein precipitation by the additional of neutral salts such as ammonium sulfate or sodium sulfate is known as salting out.
o This phenomenon is explained on the basis of dehydration of protein molecules by salts.
o This causes increased protein – protein interaction, resulting in molecular aggregation and precipitation.
o The amount of salt required for protein precipitation depends on the size (molecular weight) of the protein molecule.
o The higher is the protein molecular weight, the lower is the salt required for precipitation. Thus, serum globulins are precipitated by half saturation with ammonium sulfate while albumin is precipitated by full saturation.
o Salting out procedure is conveniently used for separating serum albumins from globulins.
o The addition of small quantities of neutral salts increases the solubility of proteins. This process called as salting in is due to the diminished protein–protein interaction at low salt concentration.
· Precipitation by salts of heavy metals:
o Heavy metal ions like Pb2+, Hg2+, Fe2+, Zn2+, Cd2+ cause precipitation of proteins.
· Precipitation by anionic or alkaloid reagents:
o Proteins can be precipitated by trichloroacetic acid, sulphosalicylic acid, phosphotungstic acid, picric acid, tannic acid, phosphomolybdic acid etc.
o By the addition of these acids, the proteins existing as cations are precipitated by the anionic form of acids to produce protein-sulphosalicylate, protein-tungstate, protein-picrate etc.
o Industrial tanning of leather is based on the principle of protein precipitation by tannic acid.
· Precipitation by organic solvents
o Organic solvents such as alcohol are good protein precipitating agents.
o They dehydrate the protein molecule by removing the water envelope and cause precipitation.
o The use of surgical spirit (about 20% alcohol) as a disinfectant is based on the precipitation of proteins and the death of bacteria.
7. Colour reactions of proteins
· The proteins give several colour reactions which are often useful to identify the nature of the amino acids present in them.
· Biuret reaction: Biuret is a compound formed by heating urea to 180°C.
· When biuret is treated with dilute copper sulfate in alkaline medium, a purple colour is obtained.
· This is the basis of biuret test widely used for identification of proteins and peptides.
DENATURATION
· The phenomenon of disorganization of native protein structure is known as denaturation.
· Denaturation results in the loss of secondary, tertiary and quaternary structure of proteins.
· This involves a change in physical, chemical and biological properties of protein molecules.
Agents of denaturation
· Physical agents: Heat, violent shaking, X-rays, UV radiation.
· Chemical agents: Acids, alkalies, organic solvents (ether, alcohol), salts of heavy metals (Pb, Hg), urea, salicylate, detergents (e.g. sodium dodecyl sulfate).
Characteristics of denaturation
1. The native helical structure of protein is lost
2. The primary structure of a protein with peptide linkages remains intact i.e., peptide bonds are not hydrolysed.
3. The protein loses its biological activity.
4. Denatured protein becomes insoluble in the solvent in which it was originally soluble.
5. The viscosity of denatured protein (solution) increases while its surface tension decreases.
6. Denaturation is associated with increase in ionizable and sulfhydryl groups of protein. This is due to loss of hydrogen and disulfide bonds.
7. Denatured protein is more easily digested. This is due to increased exposure of peptide bonds to enzymes. Cooking causes protein denaturation and, therefore, cooked food (protein) is more easily digested. Further, denaturation of dietary protein by gastric HCl enhances protein digestion by pepsin.
8. Denaturation is usually irreversible. For instance, omelet can be prepared from an egg (protein-albumin) but the reversal is not possible.
9. Denaturation is sometimes reversible (known as renaturation). Hemoglobin undergoes denaturation in the presence of salicylate. By removal of salicylate, hemoglobin is renatured.
10. Denatured protein cannot be crystallized.

Hi…!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
About The Author
Hi...!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
