Lipids
· Lipids are derived from Greek word i.e. Lipos means fats.
· Lipids are the biological molecules that are soluble in organic solvents (i.e. alcohol, ether etc.) but insoluble in aqueous solution, or potentially related to fatty acids and utilized by the living cells.
· Lipids are not polymers (because they are not build from monomers).
N.B:
· Polymers are macromolecules with high molecular weight build from repetitive units of monomers.
· Monomers are simple molecules with low molecular weight.
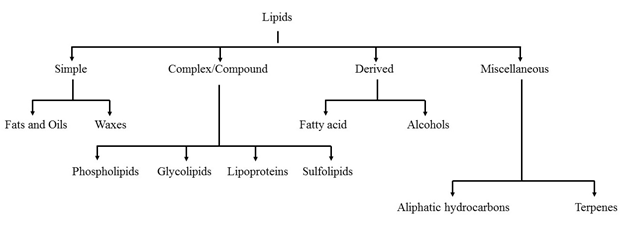
Classification of lipids
Function of lipids
· Storage of energy.
· Due to high energy value it is the important dietary compounds.
· Fats stores in adipose tissue, where it is serves as a thermal insulator in the subcutaneous tissue and around certain organs.
· Structural components of biological membranes (lipoproteins, phospholipids and sphingolipids).
· Act as a carrier of lipid soluble vitamins into cells of small intestine (A, D, E, K).
1. Simple lipids:
· These are the ester of fatty acid with alcohols.
a) Fats and oils
o These are ester of fatty acid with glycerol.
o The main difference between fats and oil is by their physical nature. Oil is liquid while fat is solid at room temperature.
b) Waxes
o Ester of fatty acid (high molecular weight) with alcohol other than glycerol.
o Long chain fatty acid.
o Waxes are hydrophobic in nature.
o Most common alcohol present in waxes is Cetyl alcohol.
o These alcohol may be aliphatic or acyclic.
2. Complex or compound lipids
· Compound lipid are ester of fatty acids with alcohol containing additional group example: phosphate, nitrogenous base, carbohydrate, protein etc.
a) Phospholipid
o They contain phosphoric acid residue and frequently have nitrogen containing base and other substituents example:
i. Glycerophospholipid
§ Contain glycerol as the alcohol.
§ Example: lecithin, Cephalin.
ii. Spingophospholipids
§ In this group of phospholipids sphingosine is the alcohol.
§ Example: Sphingomyelin.
b) Glycolipids
o Lipid containing fatty acid, carbohydrate and nitrogenous base.
o The alcohol is sphingosine, hence they are also called ad glycosphingolipids.
o Glycerol and phosphate are absent
o Example: cerebrosides, gangliosides.
c) Lipoproteins
o Macromolecular complex of lipids with proteins.
d) Others
o Sulfolipids, aminolipids, and lipopolysaccharides are under the complex lipids.
3. Derived lipid
· These substances are derived by from compound and simple lipids.
· These fatty acids includes alcohol, mono and diglycerides, carotenoids, steroids and terpenes.
4. Miscellaneous lipids
· It includes large numbers of compounds possess characteristics of lipids.
· Example: carotenoids, squalene, hydrocarbons such as pentacosane (in bees wax), terpenes etc.
Fatty acids
· Fatty acids are carboxylic acids with hydrocarbon side chain.
· It is the simplest form of lipids.
· The general formula is R-(CH2)n-COOH.
· They occur mainly as esters in natural fats and oils but do occur in unesterified form as free fatty acids.
· Fatty acids that occur in natural fats are usually straight chain derivatives containing an even number of carbon atoms as biosynthesis occurs through addition of 2 carbon atoms stepwise.
· Example: stearic acid (18C), palmitic acid (16C)
· Odd carbon fatty acid
· Example: propanoic acid (3C), Valeric acid (5C).
Occurrence of fatty acid
· Majorly occur in esterified form as major constituents of various liquids.
· They also present as free fatty acid (unesterified).
· Animal source of fatty acids are simpler in structure in contrast to those of plant origin.
· The group contains such as epoxy, keto, hydroxyl and cyclopentane rings.
Classification of fatty acids
Saturated fatty acid | Unsaturated fatty acid |
· Contain only single C-C bond. · Closely packed · Strong attractions between chains. · High melting points. · Solid at room temperature. | · Contain one or more double C-C bonds. · Nonlinear chains do not allow closely. · Few interaction between chains. · Low melting points. · Liquid at room temperature. |
· Fatty acid with one double bond are monounsaturated.
· Fatty acids with 2 or more double bonds are known as poly unsaturated fatty acid (PUFA).
Nomenclature of fatty acids
· Saturated fatty acid – suffix – anoic e.g. Octanoic acid.
· Unsaturated fatty acid – suffix – enoic – Octadecanoic acid.
Numbering of carbon atoms
· It starts from carboxy carbon – 1
· The carbon adjacent to this (carboxyl C) are – 2, 3, 4….. or alternatively α, β, ν and so on.
· Terminal carbon containing ethyl group is known as omega (ω), starting from methyl end.
· Example:
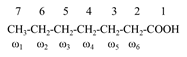
Essential fatty acids
· Essential fatty acids are cannot be synthesized by human body, therefore must be supplied in the diet.
· Chemically, they are poly unsaturated fatty acid.
· Example: Linoleic acid, linolenic acid
· If the amount of linoleic acid in diet is not sufficient then Arachidonic acid becomes essential.
Function
· Structural element of tissue.
· Structural element of gonads.
· Synthesis f prostaglandin and other compound.
· Structural elements of mitochondrial membrane.
· Serum level of cholesterol.
· Effect on clotting time
· Formation of lipoproteins.
· Prevention of fatty liver.
· Synthesis of eicosanoids.
Deficiency of EFA
· Phrynoderma or toad skin.
· Loss of hair and poor wound healing.
· Edema.
· Impaired vision.
· High triglyceride.
· High BP
· Hematologic disturbance.
· Imbalance of immunity etc.
Isomerism in unsaturated fatty acid
· Exhibit geometric isomerism.
· If the atoms or acyl groups are present on the same side of the double bond – cis configuration.

· On the other hand the groups occurs on opposite site it is a Trans configuration.

Hydroxyl fatty acid
· Example of hydroxyl fatty acid is β-hydroxybutyric acid.
· Cerebronic acid and recinolic acid are long chain hydroxyl fatty acid.
Cyclic acid
· Chaulmoogric acid (obtain from Chaulmoogra oil) contains cyclopentenyl ring.
Eicosanoids
· It is related to eicosapolyenoic fatty acids include prostaglandin, prostacyclin, leukotrienes, and thromboxane.
Triacylglycerol (TAG)
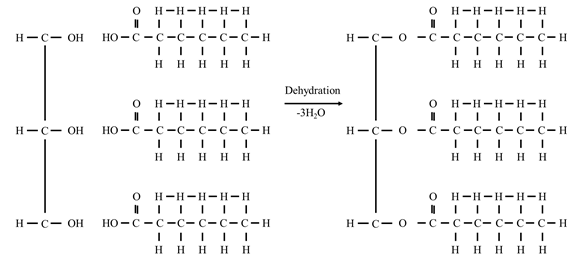
- It is also known as natural fats and triglycerides (TG).
- TAG chemically esters of glycerol with fatty acid.
- It is widely distributed in both plant and animals.
- Insoluble in water and non-polar in character.
Fats as storage fuel
- TGAs are the most abundant group of lipids.
- The primary function as fuel reservoir of animals.
- The reservoir capacity of fats in normal human about (men 20%, women 25% by weight) is sufficient to meet body’s caloric requirement for 2-3 months.
Fats primarily occurs in adipose tissues
- Adipocytes are located in subcutaneous layer and in the abdominal cavity.
- The fats are stored in the form of globules dispersed in the entire cytoplasm and TGA are not the structural components of biological membrane.
Structure of acylglycerols




Simple triacylglycerols
- Simple: contain the same type of fatty acid residue at all the three carbons
- e.g. tristearin

Mixed
triacylglycerols
- Contain 2 or 3 different types of
fatty acid residues.
- Mixed TGs are more common
- In general
- C1 – saturated fatty acid
- C2 – unsaturated
- C3 – can be either
- Triacylglycerol of plants have
higher content of unsaturated fatty acids compared to that of animals.

Properties of triacylglycerol
1. Hydrolysis
2. Saponification
3. Rancidity
4. Lipid peroxidation in vivo
Hydrolysis
- Triacylglycerols undergo enzymatic hydrolysis to finally liberate free fatty acid and glycerol.
- This process is catalyzed by lipases.
- It is important for digestion of fat in GIT and fat metabolism from adipose tissue.

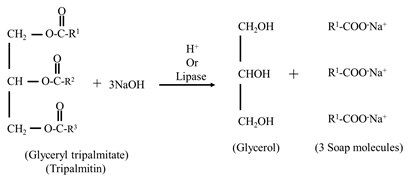
Saponification
- The hydrolysis of triacylglycerols by alkali to produce glycerol and soaps in known as saponification.
Rancidity
- It is a term used to represent the deterioration of fats and oils resulting in an unpleasant taste.
- Fats containing unsaturated fatty acids are more susceptible to rancidity
- It occurs when fats and oils are expose to air, moisture, light, bacteria etc.
- Hydrolytic rancidity occurs due to partial hydrolysis of triacylglycerols by bacterial enzymes
- Oxidative rancidity is due to oxidation of unsaturated fatty acid
- This results in the formation of unpleasant products such as dicarboxylic acids, aldehydes, ketones etc.
- They are unsuitable for human consumption
Antioxidants
- The substances which can prevent the occurrence of oxidative rancidity are known as antioxidants
- Trace amounts of antioxidants such as tocopherols (vitamin E), gallic acid an alpha naphtol are added to the commercial preparations to prevent rancidity
- Propyl gallate, butylated hydroxyanisole, and butylated hydroxy toluene are the antioxidants used in food preservation
Lipid peroxidation in vivo
- In he living cells lipids undergo oxidation to produce peroxides and free radical which can damage the tissue.
- The free radical are believed to cause inflammatory diseases, ageing, cancer etc
- It is fortunate that cells possess antioxidant like vitamin E, urate and superoxide dismutase to prevent in vivo lipid peroxidation
Tests to check purity of fats and oils
Iodine number:
· It is defined as the grams (number) of iodine absorbed by 100g of fat or oil
- Iodine number is useful to know the relative unsaturation of fats and is directly proportional to the content of unsaturated fatty acids
- Lower is the iodine number less is the degree of unsaturation
- Determination of iodine number will help to know the degree of adulteration of a given oil.
Saponification number:
· it is defined as the mg (number) of KOH required to hydrolyze one gram of fat or oil
- Saponification number is the measure of the average molecular size of the fatty acids present
- The value is higher for fats containing short chain fatty acids
Acid number:
· It is defined as the number of mg of KOH required to completely neutralize free fatty acids present in one gram fat or oil
- In normal circumstances refined oil should be free from any free fatty acids.
- Oil on decomposition due to chemical or bacterial contamination yield free fatty acids
- Oil with increased acid number are unsafe for human consumption
Phospholipids
- These are compound or complex lipids containing phosphoric acid, in addition to fatty acids, nitrogenous base and alcohol.
- Cass of phospholipids.
- Glycerophosholipid (containing glycerol as the alcohol)
- Sphingophosholipids (containing sphingosine as the alcohol)
1) Glycerophosholipids
- Glycerophosholipids are the major or main class of phospholipids that occurs in biological membrane and are important in the cell’s semipermeability.
- In presence of triglycerols and cholesterol with glycerophospholipid to increase their solubility in the blood.
- They consist of glycerol-3-phosphate esterified at its C1 and C2 with fatty acid.
- C1 contains a saturated fatty acid while C2 contains an unsaturated fatty acid.
- Each glycerophospholipids contains a polar head and nonpolar tail.
- Similar to triglycerols, but have one eater bond replaced with an amino alcohol phosphate ester.

i) Phosphatidic acid
- This is simplest phospholipid.
- It doses not occur in good concentration in tissues.
- it is an intermediate in the synthesis of TGA and phospholipids.

ii) Lecithin
- These are the most abundant group of phospholipids in the cell membrane.
- Chemically lecithin (Greek word-Lecithos-Egg yolk) is a phosphatidic acid with choline as a base.

a) Dipalmitoyl lecithin
- Found in lungs
- It is a surface acting agent and prevents the adherence of inner surface of lungs due to surface tension.
- In absence of diapalmitoyl lecithin there is a respiratory distress syndrome in infants occur.
b) Lysolecithin
• It is formed by removal of the fatty acid either at C1 or C2 of lecithin
iii) Cephalins
- Ethanolamine is the nitrogenous base present in Cephalins.

iv) Phosphatidylinositol (PI)
- Inositol is attached to phosphatidic acid to give PI.
- This is an important component of cell membrane.
- The action of certain hormone (e.g. oxytocin, vasopressin) is mediated through PI.

v) Phosphatidylserine
- The amino acid serine is attached in this group.

vi) Plasmalogens
- When a fatty acid is attached by ether linkage at C1 of glycerol is glycerophospholipids, the resultant compound is plasmalogen.

vii) Cardiolipin
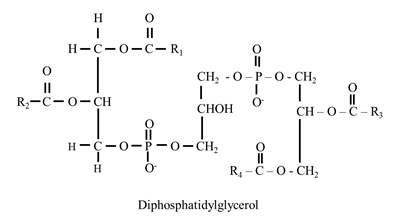
- It was first isolated from the heart muscles.
- It consist of two molecules of phosphatidic acid held by an additional glycerol through phosphate group.
- It is an important component of inner mitochondrial membrane.
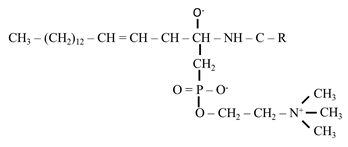
2) Sphingomyelins
- Sphingosine is attached by an amide linkage to a fatty acid to produce Ceramide
- They are the important constituents of myelin and are found in good quantity in brain and nervous tissues
- Ceramide act as a second messenger by regulating programmed cell death
- A ceramide containing 30-carbon fatty acid is a major component of skin and it regulates skin’s water permeability
Functions of phospholipids
· Phospholipids are the components of cell membrane, mitochondrial membrane and lipoproteins.
· Play a vital role in blood coagulation.
· In mitochondria phospholipid maintain the conformation of electron transport chain components. So cellular respiration takes place.
· Membrane phospholipids acts as source of Arachidonic acid.
· It helps to prevent fats accumulation in liver.
· It play a vital role in the transportation and removal of cholesterol from cells.
Glycolipids
- Glycolipids also known as glycosphingolipids are important constituents of cell membrane and nervous tissue (especially brain).
- They occur particularly in the outer leaflet of the plasma membrane, where they contribute to cell surface carbohydrates.
- They contain ceramide and one or more sugars.
- Ceramide + Glycose = Glucocerebroside
- Ceramide + Galactose = Galactocerebroside
Globosides (ceramide oligosaccharides)
- They contain two or more hexoses or hexosamines, attached to a ceramide molecule.
- Ceramide + Galactose + Glucose = Lactosyl ceramide
- Lactosyl ceramide is a component of erythrocyte membrane.
Gangliosides
- These are predominantly found in ganglions and are most complex form of glycosphingolipid.
- They are formed when ceramide oligo-saccharides have at least one or more molecule of NANA ( N-acetyl neuraminic acid ), most important (Sialic acid) attached to them.
- Ceramide – Glucose – galactose – NANA ; this is designated as GM3 (ganglioside M3).
- The most important gangliosides present in the brain are GM1, GM2, GD and GT.
- G – Ganglioside
- M, D, T – Mono, Di, Tri – sialic acid residue.
- Number denotes carbohydrate sequence attached to the ceramide.
Lipoproteins
- Lipoproteins are the molecular complexes of lipids with proteins.
- These are the transport vehicles for lipids in circulation.
- Mainly five types
- Chylomicrons – (Largest ,lowest in density due to high lipid/protein ratio: highest in triacylglycerols as % of weight)
- Very low density lipoprotein (VLDL) – (2nd highest in triacylglycerols as % of weight )
- Low density lipoprotein (LDL) – (highest in cholesterol esters as % of weight. )
- High density lipoprotein (HDL) – (highest in density due to high protein/protein ratio)
- Free fatty acid albumin complex.
Steroids
- Steroids are the compounds containing a cyclic steroid nucleus (or ring) namely cyclopentanoperhydrophenanthrene (CPPP).
- lt consists of a phenanthrene nucleus (rings A, B and C) to which a cyclopentane ring (D) is attached.
- The steroid nucleus represents saturated carbons, unless specifically shown as double bonds. The methyl side chains (19 and 18) attached to carbons 10 and 13 are shown as single bonds. At carbon 17, steroids usually contain a side chain.
- Cholesterol includes, bile acids, vitamin D, sex hormones, adrenocortical hormones, sitosterols, cardiac glycosides and alkaloids.
- lf the steroid contains one or more hydroxyl groups it is commonly known as sterol (means solid alcohol)

Cholesterol
- Cholesterol is coming from Greek word Chole – bile. First isolated from bile (solid alcohol from bile).
- Cholesterol is the most abundant sterol of animals. . It is widely distributed in all cells and is a major component of cell membranes and lipoproteins.
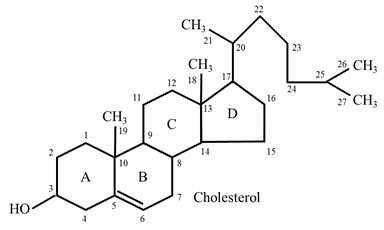
Structure and occurrence
- The structure of cholesterol (C27H46O).
- It has one hydroxyl group at C3 and a double bond between C5 and C6.
- 8 carbon aliphatic side chain is attached to C17.
- Cholesterol contains a total of 5 methyl groups.
- Due to the presence of an -OH group, cholesterol is weakly amphiphilic.
- Cholesterol is found in association with fatty acids to- form cholesteryl esters (esterification occurs at the OH group of C3).
Properties and Reactions
- Cholesterol is a yellowish crystalline solid. The crystals, under the microscope, show a notched appearance.
- Cholesterol is insoluble in water and soluble in organic solvents such as chloroform, benzene, ether etc.
- Reactions include Salkowski’s test, Liebermann-Burchard reaction and Zak’s test
Functions of Cholesterols
- Cholesterol is a poor conductor of heat and electricity.
- Present nervous tissue. Functions as an insulating cover for the transmission of electrical impulses in the nervous tissue.
- Biochemical function like: synthesis of bile acids, hormones (sex and cortical) and vitamin D etc.
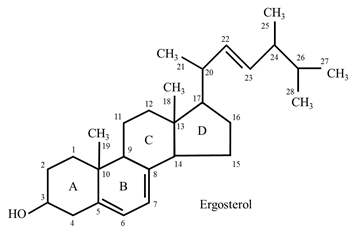
Ergosterol
- Ergosterol occurs in plants. It is also found as a structural constituent of membranes in yeast and fungi.
- It is an important precursor for vitamin D. When exposed to light, the ring B of ergosterol opens and it is converted to ergocalciferol, a compound containing vitamin D activity.
- The other sterols present in plant cells include stigmasterol and β-sitosterols.
Amphipathic
- Greek: Amphi – Both and Pathos – Passion
- Molecules which contain both hydrophobic and hydrophilic groups are known as amphipathic
- Example: fatty acids, phospholipids, sphingolipids, bile salts and cholesterol are amphipathic in nature.

Hi…!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
About The Author
Hi...!! This is Smrutiranjan Dash, Assistant Professor of Pharmacology from Odisha, India. With a passion for teaching and a dedication to advancing the field of pharmacology, I am committed to sharing knowledge, fostering innovation, and inspiring future healthcare professionals.
